In vaccine development, safety and efficacy are critical. Recent studies have shown that multiplex immunoassays offer a flexible way to monitor immune responses. With the ever-increasing pressure on scientists to develop a practical COVID-19 vaccine, multiplex immunoassays stand out as a proven tool.
The desire to prevent deaths and health complications and return to a semblance of normal pre-COVID-19 life has institutions racing to develop a safe and effective vaccine for SARS-CoV-2. The pandemic has placed the trials and tribulations of COVID-19 vaccine research and development in the spotlight.
Fortunately, research into the structure and function of SARS-CoV-2 has proceeded rapidly, due to the concerted efforts of scientists worldwide. This knowledge enables the use of diverse vaccine design strategies (summarized by the New York Times in Corum et al. 2020).
Finding novel ways to use technologies that have been previously proven to work in vaccine development is one way to further speed up the development of a COVID-19 vaccine. Among these technologies are multiplex immunoassays, which have been used to evaluate efficacy of and immune system response to vaccines for more than 15 years.
Bead-based multiplex xMAP immunoassays enable the detection of multiple analytes using the ratio of different fluorescent dyes in each bead (Figure 1A). The specified target analyte is detected based on the fluorescent intensity of the reporter molecule, phycoerythrin. These assays are run on 96-well plates and read on Bio-Plex or Luminex instruments. These readers distinguish each bead region associated with an analyte and measure fluorescence intensity, enabling the development of quantitative and qualitative assays (Figure 1B and C).
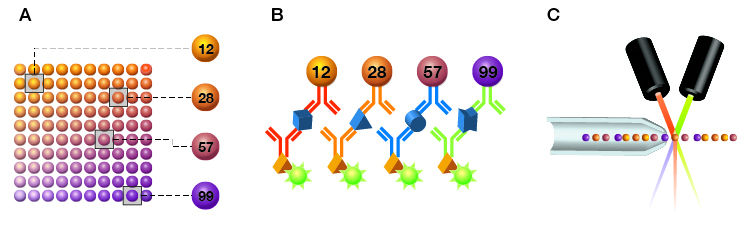
Fig. 1. Schematic representation of Bio-Plex Assays, a type of bead-based multiplex immunoassay. A, Bio-Plex Assays are designed using microscopic magnetic beads, each with a different color code (spectral address) to permit discrimination of individual assays. Beads are dyed with different ratios of two fluorophores, classifying them into up to 100 unique bead regions (xMAP technology); B, Beads are coupled to antibodies that bind specific analytes; C, Bound analytes are detected using a biotinylated antibody and quantified using streptavidin coupled to a fluorophore (phycoerythrin). Fluorescence of the beads and of phycoerythrin are measured simultaneously.
Streamlining Screening
The process of screening is necessary to ensure that only the best candidates are selected for further testing, but can be time- and resource-intensive. Multiplex immunoassays have been used throughout the vaccine research process to expedite screening.
Glaxo Smith Kline, a well-known vaccine manufacturer, utilizes a single-step cloning strategy. In this technique, Fluorescence-Activated Cell Sorting (FACS) is used to deposit single cells in 96-well plates, where clones are initially screened for robust growth through imaging. A sensitive, custom, multiplex sandwich immunoassay is then used to quantify expression of the multi-subunit viral protein used in the vaccine. This process was used to narrow 4,000 clones to less than 80 for passaging — about 80% fewer than with traditional methods, saving resources and shaving weeks off the development process (Sargent 2020).
Aiding Adjuvant Selection
Multiplex immunoassays have also been used in adjuvant selection during vaccine development. The high throughput and small volume requirements of multiplex immunoassays makes them ideal for these types of studies, where multiple experimental conditions, tissues, and timepoints must be monitored.
For example, Bio-Plex Pro Mouse Cytokine Assays were used to monitor the effects of adjuvants in influenza vaccines in serum and lung homogenate of mice of varying ages (McDonald et al. 2017). The study found that cytokine response varied depending on age and whether an adjuvant was included. They also found that cytokine increases following infection in vaccinated mice were localized, rather than systemic.
In another study, Bio-Plex Pro Human Cytokine Assays were run on cell culture supernatant samples to evaluate cytokine profiles related to stimulation from different influenza antigens (Nakayama et al. 2018). Cytokine levels were studied in conjunction with seroconversion pre- and post-vaccination to understand whether there were predictive cytokine biomarkers that correlated with developing acquired immunity to an influenza vaccine.
From Animal Models to Clinical Trials (and Beyond)
Another format that has been useful in evaluating vaccine immunogenicity is the indirect multiplex immunoassay. In this technique, multiple antigens are bound to different beads, allowing antibodies specific to the bound pathogen proteins to be measured in serum and plasma samples. Serological studies can be used through the entire continuum of vaccine research and development, spanning animal models in research, the preclinical phase, clinical trials, and even post-market surveillance studies.
There have been several studies using serological multiplex immunoassays to monitor efficacy of Gardasil, a vaccine for Human papilloma virus (HPV). In one such study, viral subunits used in the vaccine were coated on beads and used to quantify immune response with and without adjuvant formulation in non-human primate models (Ruiz W et al. 2005). This study showed that the inclusion of an adjuvant resulted in higher antibody titers.
In the same study, a competitive assay was developed, using beads coated with known neutralizing epitopes to detect levels of neutralizing antibodies in samples after vaccination (Ruiz W et al. 2005). The competitive assay format was used for human serum in clinical trials, demonstrating that seroconversion and protective immunity were achieved with Gardasil. The multiplex competitive assay format enabled the detection of neutralizing antibodies to Gardasil’s multivalent vaccine, thereby increasing testing throughput (Smith et al. 2005).
The utility of the competitive assay format was also extended to post-market surveillance ten years post-vaccination, assessing the efficacy, immunogenicity, and safety of Gardasil. The results from this assay showed that seroconversion was achieved to all four quadrivalent HPVs with peak titers after the third dose. Of the thousands of patients tested, all had high levels of antibodies against the four virus-like particles, implying a sustainable and strong immunological response along with the ultimate confirmation of being negative for HPV infections.
Learn More about Multiplex Immunoassays
The flexible assay formats along with the time and sample savings achieved when using multiplex immunoassays in vaccine research and development are well established. This technique may prove useful for confirming the efficacy and profiling of the immune response to COVID-19 vaccines currently under development.
Click here to read more about Bio-Plex Assays in COVID-19 vaccine research.
References
Corum J et al. (2020). Coronavirus Vaccine Tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html, accessed November 30, 2020.
McDonald JU et al. (2017). Inflammatory responses to influenza vaccination at the extremes of age. Immunology 151, 451–463.
Nakayama T et al. (2018). Cytokine production in whole-blood cultures following immunization with an influenza vaccine. Hum Vaccin Immunother 14, 2,990–2,998.
Ruiz W et al. (2005). Kinetics and isotype profile of antibody responses in rhesus macaques induced following vaccination with HPV 6, 11, 16 and 18 L1-virus-like particles formulated with or without Merck aluminum adjuvant. J Immune Based Ther Vaccines 3, article 2.
Sargent B (2020). Single-Step Cloning Strategy Speeds Timeline for Novel Vaccine Candidates. https://cellculturedish.com/single-step-cloning-strategy-speeds-timeline-for-novel-vaccine-candidates/, accessed November 30, 2020.
Smith JF et al. (2008). Evolution of type-specific immunoassays to evaluate the functional immune response to GARDASIL, a vaccine for Human Papillomavirus types 16, 18, 6, and 11. Hum Vaccin 4, 134–142.

