Longitudinal SARS-CoV-2 antibody profiling elucidates the longevity of the humoral immune response to COVID-19 infection, evaluates seroprevalence in different populations, and is used in characterizing vaccine immunogenicity postvaccination.
The research use only Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel is a qualitative multiplex immunoassay kit that quickly and efficiently detects IgG antibodies against four SARS-CoV-2 antigens, including the nucleocapsid protein (N), receptor binding domain (RBD), spike 1 subunit (S1), and spike 2 subunit (S2) (Figure 1).
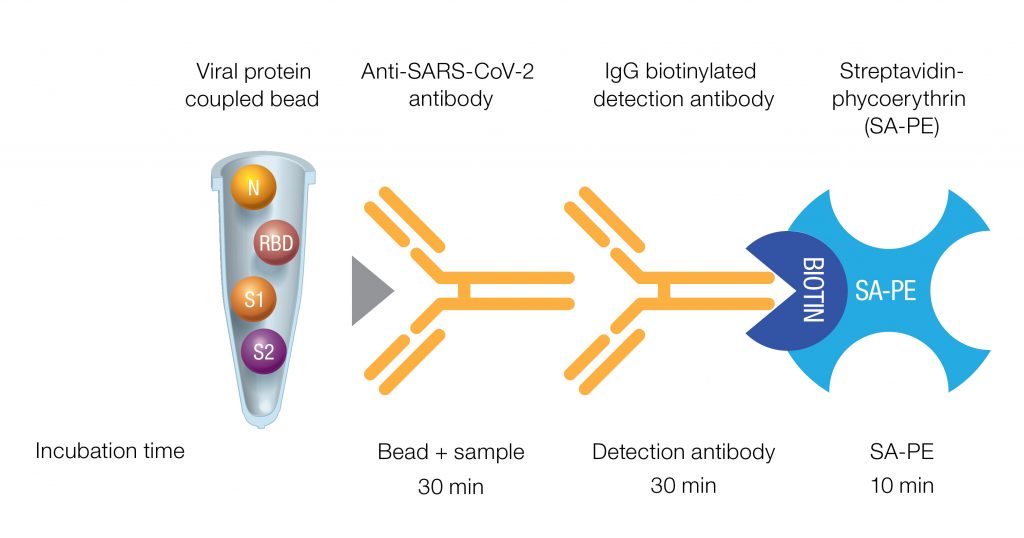
Fig. 1. Qualitative Bio-Plex Pro SARS-CoV-2 Assay format and incubation times. Four SARS-CoV-2 viral antigens (N, RBD, S1, and S2) were coated individually on magnetic beads to capture antibodies specific to these proteins. Simultaneous antibody response profiling against each viral antigen through a biotinylated detection antibody against IgA, followed by binding of SA-PE, results in an MFI readout that can be interpreted as positive or negative through assigned cutoff values. MFI, median fluorescence intensity.
These assays enable researchers to study the humoral response to COVID-19. Public health researchers can use the assays to perform seroprevalence studies that can then be used to determine the exposure rates to SARS-CoV-2 in a population, community, or institution. Since antibodies are present beyond active infection, detecting antibody levels can provide an accurate measure of exposure rates because it will include those in the population who may not have been diagnosed due to having mild or no symptoms during COVID-19 infection.
For vaccine developers, assessing the immunogenicity of their vaccine is key to proving therapeutic efficacy. Longitudinal studies evaluating antibody response are a tool for determining the immunity conferred by vaccination as well as the overall longevity of the humoral response in natural infection.
Detectable IgG levels are confirmed based on median fluorescence intensity (MFI) cutoff values (Figure 2).
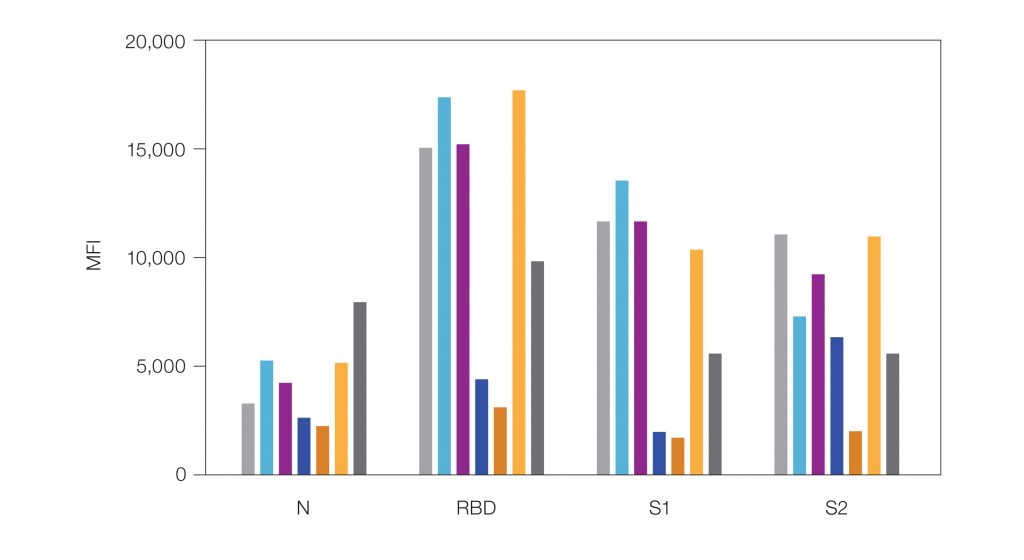
Fig. 2. Bio-Plex Pro Human IgG SARS-CoV-2 4-Plex Panel sample data. Six COVID-19 positive serum samples were assayed to evaluate levels of anti-SARSCoV-2 IgG antibodies to N, RBD, S1, and S2 viral antigens. MFI levels vary for each sample, indicating varying antibody response profiles of each patient to each of these viral antigens. MFI cutoff values indicate the samples are positive for IgG antibodies against SARS-CoV-2. MFI cutoff: >450 (N); >250 (RBD); >250 (S1); >750 (S2). Positive control (■); sample 1 (■); sample 2 (■); sample 3 (■); sample 4 (■); sample 5 (■); sample 6 (■); negative control (■; not visible on the graph because all negative control MFIs are <25). All controls and samples had a coefficient of variation (CV) <6%, with an average CV of 1.7%. MFI, median fluorescence intensity.
The product comes as a ready-to-use, 96-well kit containing premixed magnetic capture beads, an IgG-specific detection antibody, positive control, negative control, and buffers that produce results for four anti–SARS-CoV-2 antibodies using only 5 microliters of sample. Flexible configurations include singleplex assay reagents, which enable measurement of specific antibodies to viral proteins of interest. The coupled beads can be used to measure antibodies in any species, making the assay useful for both preclinical and clinical trials. The kit is compatible with research use only (RUO) Bio-Rad platforms, including the Bio-Plex 200 and Bio-Plex 3D Multiplex Immunoassay Systems, as well as all other RUO xMAP platforms.
Click here for more information about the Bio-Plex SARS-CoV-2 Assays.
Additional Reading
Sahin U et al. (2020). COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599.
Fenwick C et al. (2021). Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 95, e01828-20.

