The ability to characterize the target of cell-mediated immune response is crucial during vaccine development. Discover how the ZE5 Cell Analyzer was used to detect activation-induced markers, measure intracellular cytokines, and identify protein-specific antibodies in a rapid, high-throughput manner.
How Are Vaccines Developed?
To develop a safe and effective vaccine, knowledge of the pathogen’s structure, its mechanism of entry, and how infection spreads throughout the body are required. This knowledge contributes to vaccine antigen selection by providing a basis for how the body’s immune system responds during infection.
The optimal vaccine antigen candidate will mimic one of these attributes, giving the immune system a detailed preview of the infectious agent. This sparks the generation of neutralizing antibodies to the antigen(s). During vaccine formulation, selected antigens are monitored for stability, safety and efficacy, including immunogenicity, during preclinical and clinical trials (Cunningham et al. 2016).
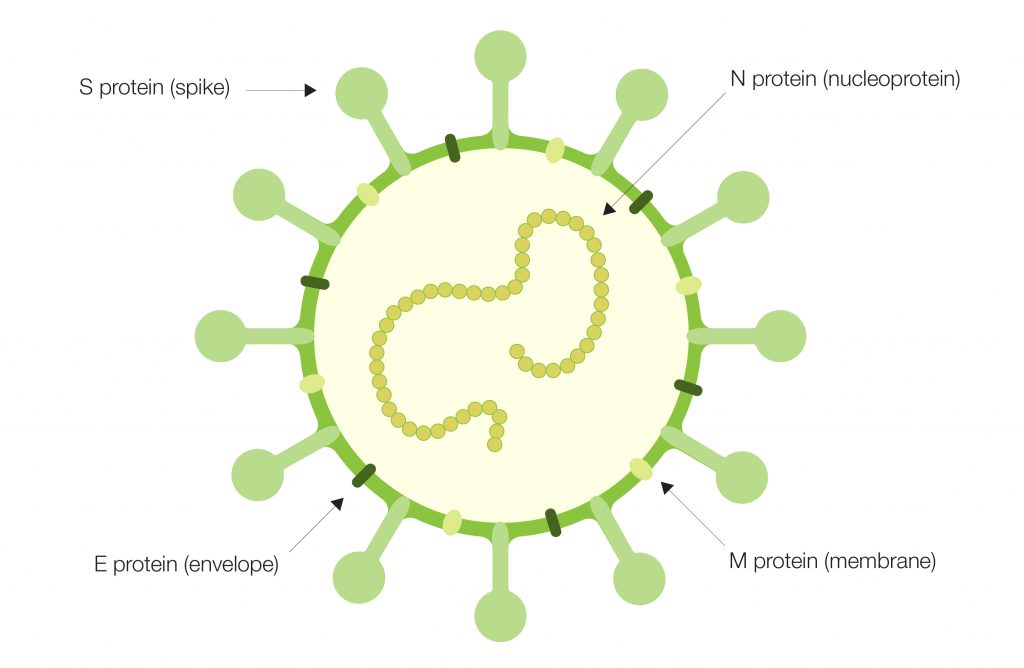
The Importance of the SARS-CoV-2 Spike Protein
It is also crucial in vaccine development to characterize the target of the cell-mediated immune response. During the COVID-19 pandemic, scientists at University of Washington (Seattle, U.S.) and Humabs BioMed SA (Switzerland) collaborated to study SARS-CoV-2 pathogenesis in humans.
The team identified neutralizing antibodies from convalescent patients with COVID-19 and ascertained that ~90 percent of the antibodies were associated with the receptor-binding domain (RBD) of the spike (S) protein on the surface of the virus, indicating its importance for virus transmission and disease progression. One neutralizing antibody specifically locked the RBD in an open conformation, providing additional structural context for vaccine antigen design (Pinto et al. 2020).
Interestingly, it has been noted that COVID-19 disease progression differs among patients primarily based upon age. Younger patients, children, and adolescents rarely develop significant symptoms, and even fewer develop complications from these symptoms. To test for humoral immunity, two separate studies examined the sera of patients who had not previously been infected with SARS-CoV-2, as well as serum samples collected prior to the onset of the pandemic.
In both studies, cross-reactive antibodies specific for the S protein were detected and postulated as the reason behind the heterogeneity observed in disease progression (Mateus et al. 2020, Ng et al. 2020). These data reiterate the importance of the S protein in both transmission and immunity.
Flow Cytometry Enables Rapid Immunology Research
In all the studies mentioned above, the Bio‑Rad ZE5 Cell Analyzer was used to either detect activation-induced markers, measure intracellular cytokines, or identify S protein–specific antibodies in a rapid, high-throughput manner.
In fact, the Francis Crick Institute (England) established a robust serology pipeline using the ZE5 Cell Analyzer to facilitate screening of serum antibodies reactive to SARS-CoV-2 antigens (Russell et al. 2020). In that study, HEK 293T cells expressing SARS-CoV-2 S protein subunits were incubated with participant serum and stained with fluorescence-conjugated anti-IgG, IgM, and IgA antibodies before high-throughput screening was performed on the ZE5 Cell Analyzer.
An additional study performed to understand the potential infection rate of frontline healthcare workers in London, the ZE5 Cell Analyzer was utilized alongside RT-PCR testing for SARS-CoV-2 as part of a robust serological testing pipeline (Houlihan et al. 2020). This displayed the versatility and speed of flow cytometry technology coupled with automation in solving key challenges in immunity surveillance.
The Importance of Immunology
The rapid development of COVID-19 vaccines could not have been accomplished without established knowledge of the S protein’s role during coronavirus pathogenesis. It was equally important to identify the role of the protein’s subdomains in neutralizing this novel virus, which required probing and analyzing the patients’ immune response to the virus.
While nucleic acid vaccine technology platforms have evolved to be able to respond to the rapid pace of vaccine development in a pandemic, they would not have been as effective if researchers could not quickly identify the best and most effective antigen — the RBD of the S protein.
For more on vaccine development tools from Bio-Rad, check out our Immunology Solutions for Coronavirus Research page.
References
Cunningham AL et al. (2016). Vaccine development: From concept to early clinical testing. Vaccine 34, 6,655–6,664.
Houlihan CF et al. (2020). Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 396, e6–e7.
Mateus J et al. (2020). Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94.
Ng KW et al. (2020). Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370, 1,339–1,343.
Pinto D et al. (2020). Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV2 antibody. Nature 583, 290–295.
Russell E et al. (2020). Adapting to the coronavirus pandemic: Building and incorporating a diagnostic pipeline in a shared resource laboratory. Cytometry 99, 90–99.