Medicines called biologics are transforming the healthcare landscape by offering hard-to-treat patients a real treatment benefit. As targeted therapies, they have brought us much closer to the realization of personalized solutions to our health problems. A biologic or biological drug is manufactured using living organisms, a different process from that used for small molecule drugs, which are typically produced via chemical synthesis. Biologics have seen a rapid rise in popularity as treatment options for many diseases; even offering cures where none existed before. Why is this true? What do biological drugs offer that other therapies do not?
Novel Therapeutics Called Biologics
Biologics are not a new class of drugs, as biological drugs such as insulin have been available for decades. What changed is the availability and potential applications for biological drugs. Our increased understanding of the biological processes that are part of human health and the changes that occur during disease or chronic illness have helped spur the development of novel biological drugs targeted to provide treatment and relief for patients (Morrow 2004). Biologics are large, complex molecules consisting of proteins, peptides, nucleic acids, sugars, or other cellular structures, or a combination of these produced within living cells or microorganisms. The shifting focus toward biological drug products results in targeted treatments that have mechanisms of action previously unavailable from existing small molecule drugs.
Both small and large molecule drugs interact with a patient’s biology; however, small molecule drugs work as inhibitors disrupting a process ordinarily associated with a particular disease. As small molecules that can penetrate cells, the potential exists for off-target interactions that cause a myriad of side effects. Large biologic drugs, in contrast, are designed to bind to specific targets with extreme precision. Sometimes, they can stimulate the immune system to react to the presence of a problem such as a tumor. Biologics target molecular processes that small molecule drugs cannot.
As healthcare requirements around the world have evolved due to the rise in aging populations and an increase in countries prioritizing the health of their citizens, research funding for diseases and treatment options has also accelerated. Recently developed biological drugs, for example, Abbvie’s HUMIRA and Roche’s RITUXAN, play a critical role in the management of many serious illnesses, including rare genetic disorders, autoimmune diseases, and cancer (Figure 1; Gottleib 2018). Often they are the only available treatment option for the patient.
In terms of approvals for novel medicines, biological drugs have grown to comprise approximately one-third of all new medicines approved by the U.S. FDA (Gottleib 2018). In addition, more than half the drugs currently in development by pharma and biotech companies are biologics. Cancer research receives the most R&D funding for development of novel biological drugs with rare diseases, autoimmune disorders, and neurological disorders occupying the next three spots (Hooven 2017).
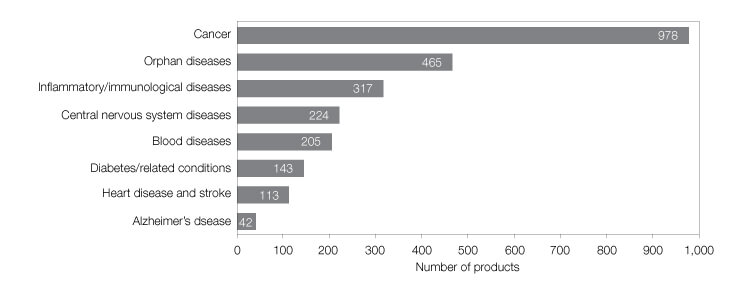
Fig. 1. Therapeutic focus of the biologics in clinical development. Adapted from Hooven 2017.
Focus on Monoclonal Antibodies
Monoclonal antibody (mAb) biological drugs top the list when compared to other biological drug types, such as proteins, enzymes, and vaccines. In addition to an increase in spending on R&D for biological drugs, supportive government initiatives and demand for personalized medicines are contributing to the expectation that the global market size for mAbs will be worth $138 billion by 2024 (Grand View Research 2016).
Manufacturing Monoclonal Antibodies
In development of a biological product, the resulting product is dependent upon the manufacturing process, which is time consuming, challenging, expensive, and often complicated. In addition to the usual clinical trials required to establish safety and efficacy, development includes a complex manufacturing process that includes genetically engineering a cell to produce a protein, testing to ensure quality, and harvesting, purifying, and stabilizing the protein (Jallal 2017).
The process of manufacturing a fragile, sensitive biological molecule, such as an antibody made from living cells, has complex requirements that include fermentation, aseptic processing and purification, storage, and testing. The active component of the drug, often a portion or modification of the original protein or polypeptide may not be clearly characterized. Due to the heterogeneous nature of the molecule, the biologic can have an impurity profile that varies with the manufacturing and testing process utilized. One of the primary confounding issues is the need to identify a purification strategy that produces the highest yield while maintaining the purity of the final product. This critical step in the process is often accompanied by very significant costs.
Recent advances in upstream processes have improved mAb titers in mammalian cell culture. Increases in the fermentation volume and protein mass have increased the challenges involved in processing harvested material. As purification processes have improved and yields have grown, the incidence of impurities has also grown and had a negative impact on the purity of the final product. In addition, elevated process and product related impurities from prolonged fermentation and higher cell density are further complications (He et al. 2011).
For downstream mAb purification, column chromatography is normally preferred using a two- to three-step process that includes capture, intermediate, and polish steps (Figure 2) and selecting specific resins based on their technical parameters. But not all resins are created equal. The ideal strategy can be customized according to final intended use and include considerations for cost, harsh elution conditions, desired monomer recovery, and purity.
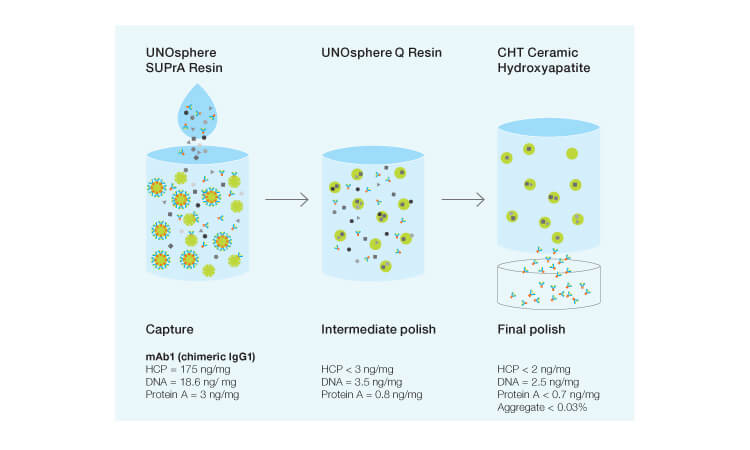
Fig. 2. Example of a mAb purification workflow.
Protein A chromatography has been the method of choice for the initial step of purification because it is relatively easy to use without modification and has the ability to remove host cell proteins, nucleic acids, endotoxins, and viruses. However, Protein A is an immunotoxin and capable of forming neutralizing antibodies (Gagnon et al. 2006). Protein A is also quite expensive. An ion exchange resin such as Nuvia S provides an economical and effective alternative to Protein A for the capture step. Nuvia S is an ultra-high capacity cation exchange (CEX) resin that provides high dynamic binding at a broad range of pH and conductivity with low backpressure at high flow rates. High flow rates minimize the mAb’s exposure to the proteases and nucleases that exist in cell culture.
Nuvia HR-S, another high-resolution CEX resin, can be used for both the intermediate and polish purification steps. It is ideal for separation of closely related biomolecules and impurities like mAb aggregates (Khandelwal 2016). Nuvia cPrime, a hydrophobic CEX resin, has unique selectivity that uses hydrophobic and CEX interaction modes to achieve effective purification in the intermediate and final polish steps. Its particle size is optimized to deliver exceptional flow properties, fast mass transfer, and stability.
The gold standard for aggregate removal is CHT Ceramic Hydroxyapatite, a highly effective mixed-mode media. CHT removes aggregates from mAbs without effecting purity or yield of monomer mAbs (He 2015). CHT binds to biomolecules by calcium metal affinity, phosphoryl cation exchange interactions, and hydrogen bonding.
Conclusion
Biologics are becoming the primary focus for a growing number of therapeutic developers. Although they are both challenging and expensive to develop, they have applications for a wide range of diseases that are not being adequately addressed with existing medicines. In addition, biologics are equally as challenging to copy since they are produced in living cells. As a result, the effort devoted to developing a successful biologic is paying off. The success of a mAb in particular depends upon its purification strategy and the effectiveness of downstream removal of aggregates.
References
Gagnon P et al. (2006). A ceramic hydroxyapatite–based purification platform: simultaneous removal of leached Protein A, aggregates, DNA, and endotoxins from mAbs. Bio-Rad Bulletin RP0033.
Gottleib S (2018). Capturing the benefits of competition for patients. fda.gov/NewsEvents/Speeches/ucm599833.htm, accessed December 11, 2018.
Grand View Research (2016). Monoclonal antibodies (mAbs) market size worth $138.6 billion by 2024. grandviewresearch.com/press-release/global-monoclonal-antibodies-market, accessed December 11, 2018.
He X et al. (2011). A purification strategy for clinical-grade monoclonal antibody using hydrophobic cation exchange chromatography. Bio-Rad Bulletin 6241.
He X (2015). Mixed-mode chromatography for mAb S aggregate removal: Comparison of CHT Ceramic Hydroxyapatite, Capto adhere, and Capto adhere ImpRes. Bio-Rad Bulletin 6749.
Hooven MD (2017). Opportunities and challenges in biologic drug delivery. americanpharmaceuticalreview.com/Featured-Articles/345540-Opportunities-and-Challenges-in-Biologic-Drug-Delivery/, accessed December 11, 2018.
Jallal B (2017). Realizing the promise of biologics. hhpronline.org/articles/2017/4/9/realizing-the-promise-of-biologics, accessed January 4, 2019.
Khandelwal P (2016). Practical guide: selecting optimal resins for monoclonal antibody process purification. Bio-Rad Bulletin 6875.
Morrow T (2004). Defining the difference: what makes biologics unique. Biotechnol Healthc 1, 24–29.
Bio-Rad and CHT are trademarks of Bio-Rad Laboratories, Inc. in certain jurisdictions. All trademarks used herein are the property of their respective owner.

