
Department of Medical Biology
Kocaeli University, Izmit, Turkey
Research Background
Our laboratory is currently focusing on the development of novel approaches for the synthesis of commercially significant human proteins. The approaches we have developed allow us to produce these proteins with high efficiency and low cost. One of the goals of producing highly purified and correctly modified proteins is to use them in the production of highly specific antibodies. The practicality and speed of the purification process was of the utmost importance to us. Previously, we had used several different technologies to speed up our purification protocols, but they did not produce the desired outcome. Recently, we have been using Bio-Rad’s medium-pressure NGC™ Liquid Chromatography System, which has allowed us to perform fully automated protein purification experiments.
Application
Our primary application with the NGC System is to purify recombinant proteins as well as the antibodies produced targeting those in-house–produced recombinant proteins. The NGC System is an efficient tool for saving time and effort when optimizing purification protocols. The method templates available in ChromLab™ Software provided with the NGC System are a good starting point when setting up a purification. The experiments can be fine-tuned and the conditions used in optimization studies can be saved without additional effort to obtain optimized protocols that yield highly purified proteins. In a recent study, we focused on the expression and purification of a cardiac marker, troponin I (cTnI), using the NGC System (Selimoğlu et al. 2016). After expressing the protein in E. coli, we optimized a purification protocol and obtained highly purified cTnI. The purified protein was then used to develop cTnI-specific antibodies and an ELISA assay detecting 1.5 pg of cTnI in human serum (Figures 1 and 2).
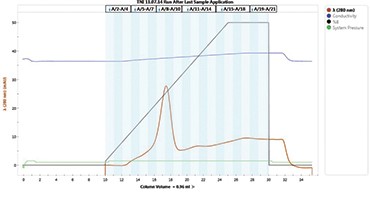
Fig. 1. Elution profile of cTnI from the IMAC column. The elution peak for cTnI was shown with a diagonal arrow.
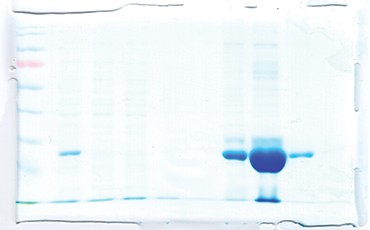
Fig 2. SDS-PAGE analysis of the cell-free extract and fractions obtained from IMAC purification of cTnI under denaturing conditions. Lane M, protein molecular weight marker; Lanes 1, Flow through; 2, Wash 1; 3, Wash 2; 4-5- 6 and 7, Fractions collected from the column.
Conclusion
The NGC System allows us to purify proteins with great ease to a level we could hardly approach previously. The ability to easily fine tune our purification protocols allowed us to generate highly purified proteins with minimal sample loss. We showed this in our recent study where we were able to purify 25 µg of highly pure troponin I per ml of E. coli culture.
Reference
Selimoğlu SM et al. (2016). Overcoming difficulties on synthesis of cardiac troponin-I. Prep Biochem Biotechnol [published online ahead of print April 12, 2016]. Accessed June 9, 2016.

