Nuvia HP-Q is a high-performance strong anion exchange resin designed for purification of large biomolecules such as plasma proteins, IgA and IgM, viruses, VLPs, and PEGylated proteins. Its optimized particle and pore size offers high dynamic binding capacity (DBC) at fast flow rates without excessive backpressure and fast mass transfer kinetics, thereby delivering excellent target recovery and improved process economics. It’s robust and stable base bead provides the high-throughput purification (HTP) demanded by today’s process manufacturing.
High Target Recovery
Why will you achieve high target recovery with Nuvia HP-Q?
Its pore size is optimized for easy accessibility and adsorption of large biomolecules, and the internal spacer length and ligand density facilitate efficient binding of the biomolecules even at high flow rates (Figure 1). Traditional ion exchange (IEX) resins don’t offer this advantage.
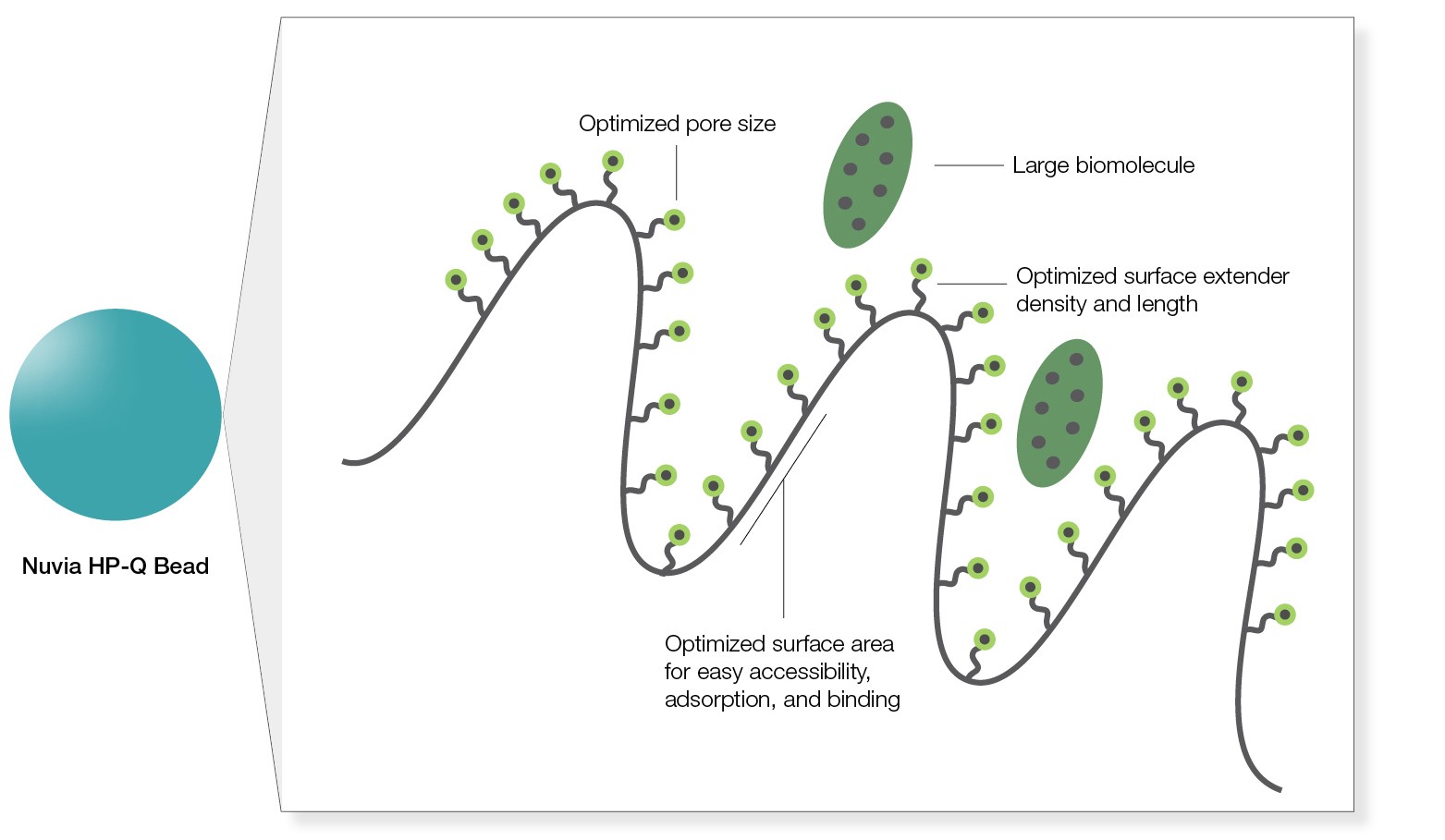
Fig. 1. Easy accessibility of large molecules for binding.
In large biomolecule purification, how does Nuvia HP-Q compare to other commercially available resins?
Nuvia HP-Q is designed to overcome the issues faced when purifying large biomolecules with other commercially available resins and help in downstream purification of such biomolecules at fast flow rates without loss in DBC and recovery.
IgM obtained from plasma fractionation showed DBC in the range of 20–25 mg/ml with Nuvia HP-Q. In contrast, other resins showed lower DBC or achieved the same DBC at a much lower flow rate (Table 1).
Table 1. Superior DBC of Nuvia HP-Q at a high flow rate relative to other comercially available resins.
| Resin | Matrix Material | Particle Size, µm | Pressure, bar | Recommended Flow Rate, cm/hr1 |
DBC (IgM) |
| Nuvia HP-Q | UNOsphere epoxide | 50 | <3 | 300 | +++2 |
| Resin 1 | Dextran beads | 50 | <3 | 30 | +++2 |
| Resin 2 | Agarose | 75 | <3 | 300 | +3 |
| Resin 3 | PS/DVB | 50 | <3 | 300 | +2 |
| Resin 4 | PMMA | 50 | <3 | 300 | +2 |
1 Recommended flow rate for industrial scale column (D > 30 cm).
2 DBC data at 10% breakthrough.
3 Data obtained from vendor presentation.
Parallel runs of thyroglobulin on Nuvia HP-Q and other commercially available resins demonstrate that Nuvia HP-Q shows best-in-class DBC and overcomes the compromised productivity issue that results from low DBCs of other IEX resins (Figure 2).
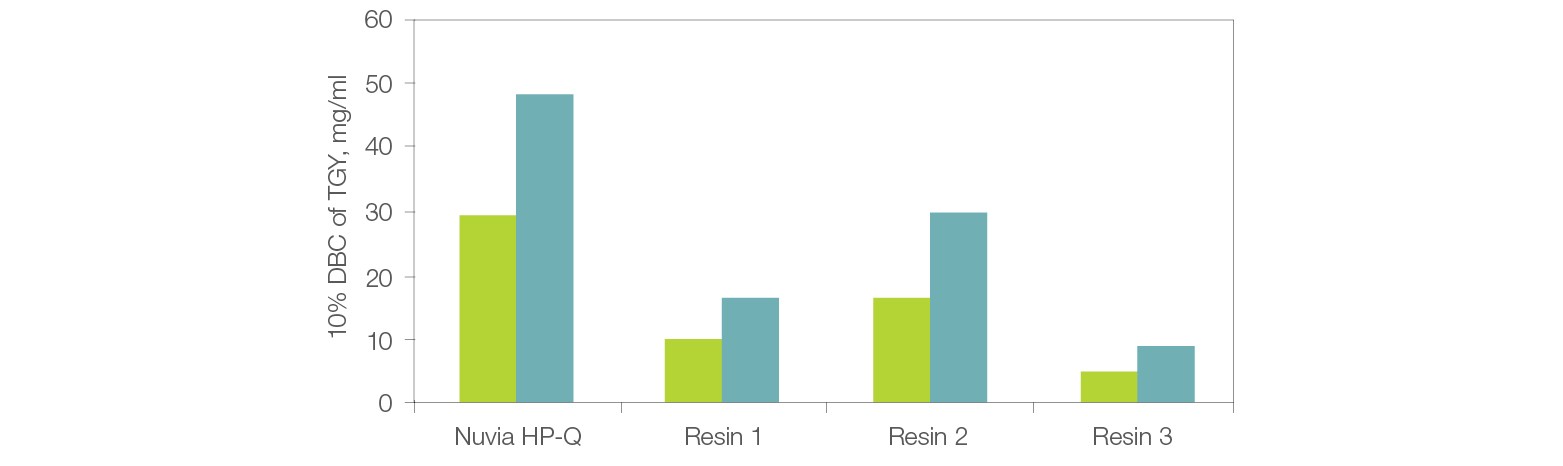
Fig. 2. Dynamic binding capacity (DBC) vs. residence time of Nuvia HP-Q. Comparison of DBCs between Nuvia HP-Q and three commercially available resins at 1.2 and 2.4 min residence times. The resins were packed into 1 ml columns. Thyroglobulin (TGY) solution (1.1 mg/ml) in 20 mM Tris Cl, pH 8.0 was loaded onto the columns until 10% breakthrough was observed. 1.2 min residence (■); 2.4 min residence (■).
High-Throughput Purification
Why will you achieve high-throughput purification with Nuvia HP-Q?
Nuvia HP-Q is built on the rugged and hydrophilic UNOsphere epoxide base bead, which does not collapse under the pressure developed due to the fast flow rate required for HTP purification (Figure 3).
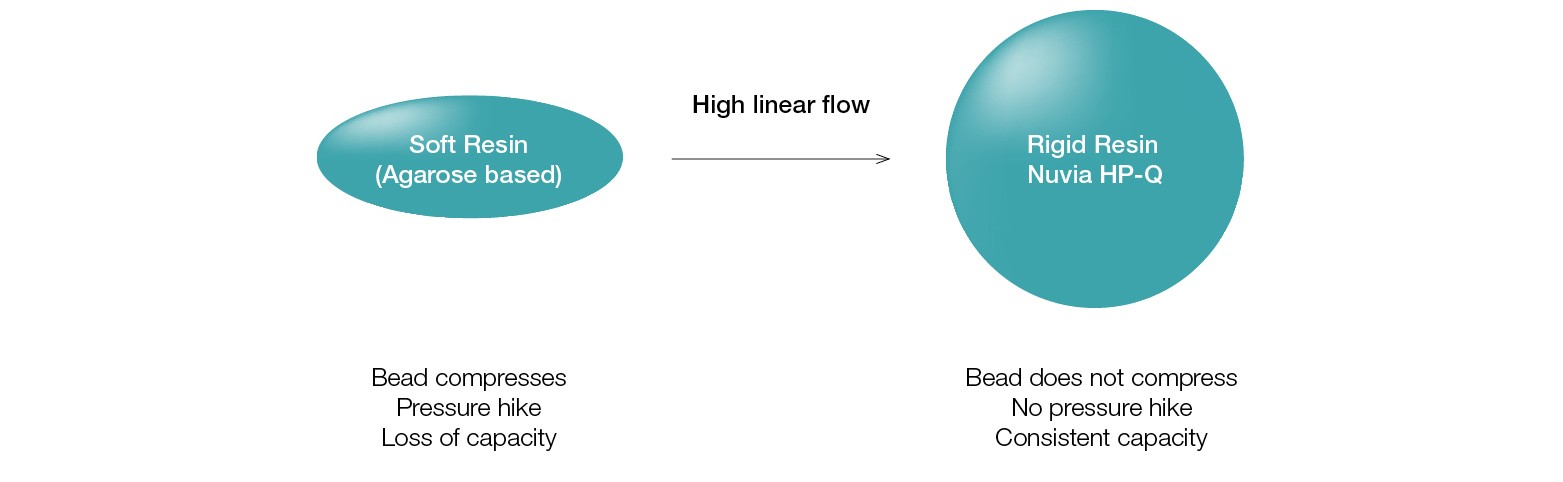
Fig. 3. Mechanically stable base bead allows HTP purification.
Can Nuvia HP-Q maintain low column pressure at the flow rates required for process purification?
The Nuvia HP-Q Resin is designed with an optimal bead size to achieve both laboratory- and process-scale purification of large biomolecules at high flow rates without being limited by column pressure. This leads to an increase in productivity during protein purification. The column pressure remains below 1.5 bar at a linear velocity of 350 cm/hr (Figure 4).
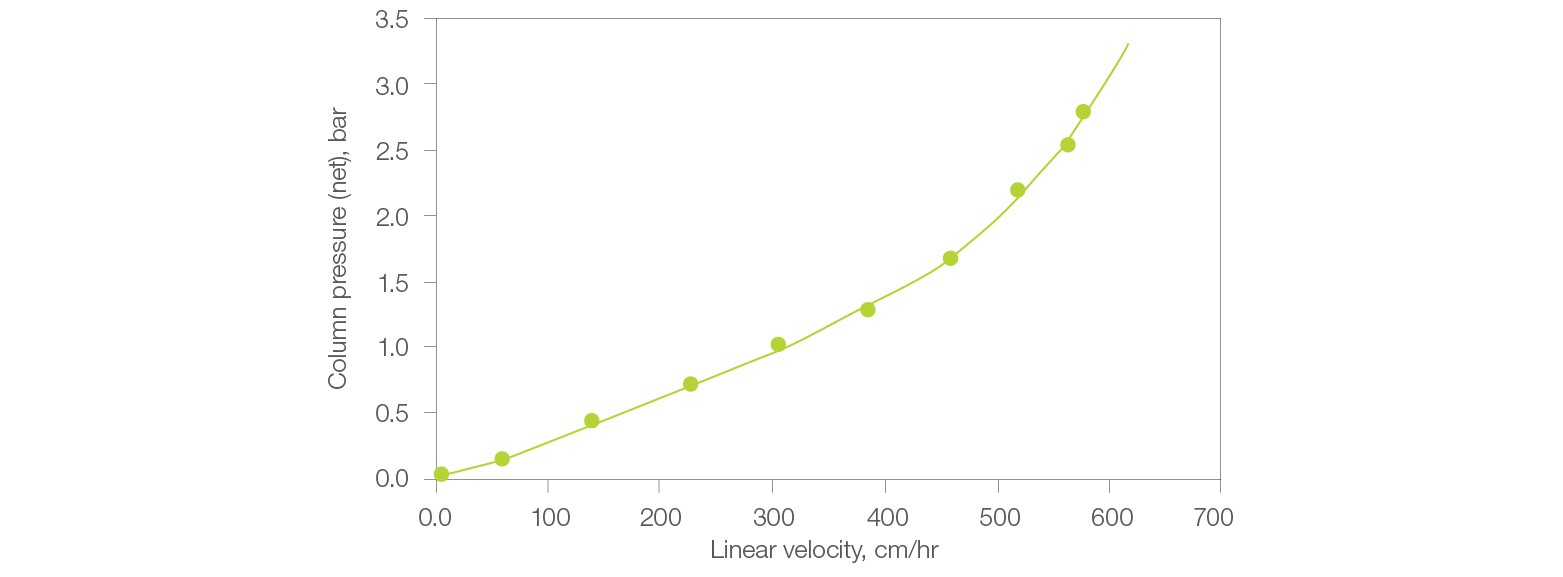
Fig. 4. Pressure/flow performance of Nuvia HP-Q Resin. Nuvia HP-Q slurry prepared in 1x PBS, pH 7.5 was packed into a 20 x 20 cm column by axial compression with a compression factor of 1.2.
Can Nuvia HP-Q be used multiple times for cost efficiency?
The chemical stability of Nuvia HP-Q allows the resin to perform consistently with minimal changes to DBC or recovery even after prolonged exposure to NaOH (Figure 5). In addition, it is produced by a validated manufacturing process that ensures batch-to-batch reproducibility.
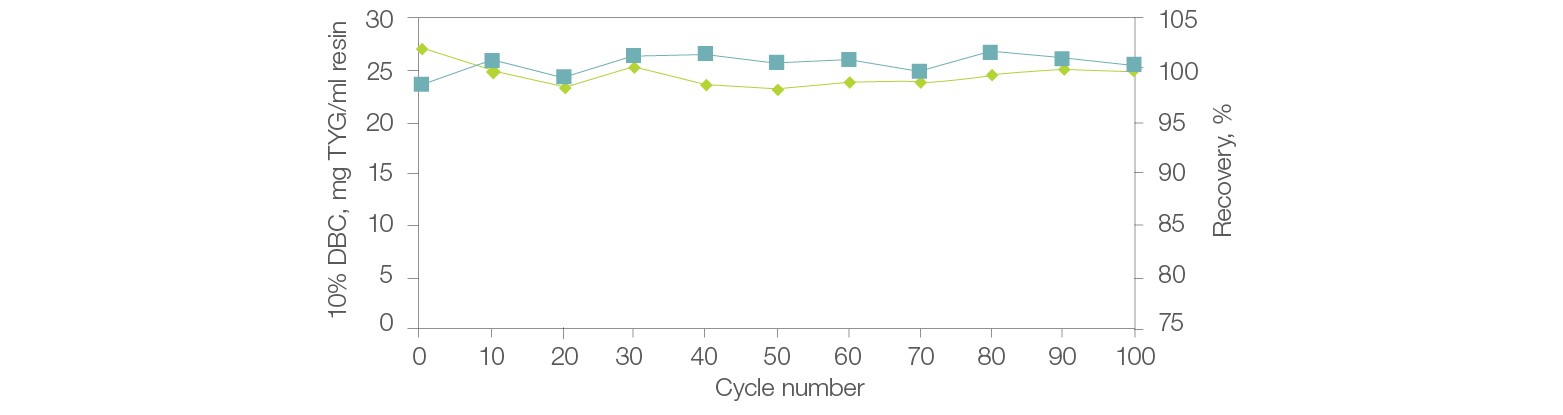
Fig. 5. Stability, reusability, and recovery with Nuvia HP-Q Resin. Thyroglobulin (TYG) solution (1.1 mg/ml) in equilibration buffer (20 mM Tris Cl, pH 8.0) was loaded onto a 1 ml Bio-Scale Mini Column (0.56 x 4 cm) packed with Nuvia HP-Q to a compression factor of 1.2. The column was operated at 300 cm/hr. The protein was eluted in 5 CV of elution buffer (20 mM Tris Cl, 1 M NaCl, pH 8.0) at 300 cm/hr. Cleaning in place (CIP) was performed on the column with 3 CV of 0.5 N NaOH at 300 cm/hr followed by a 40 min hold. The 10% DBC at linear velocity of 100 cm/hr was determined after every 10 cycles. DBC (—); recovery (—).
Is Nuvia HP-Q available in different formats for easy scalability?
Nuvia HP-Q is available in multiple user-friendly formats, including prepacked Foresight Columns and Plates for purification condition screening and bulk bottles for pilot- to manufacturing-scale purifications. In addition, it is backed by our regulatory support documentation and security of supply commitment.
In summary, Nuvia HP-Q offers the following features to improve your large molecule purification. For all its technical features, refer to bulletin 7078.
- Ideal for large molecule purification – for overcoming drawbacks of traditional IEX resins that have kinetic limitations due to non-optimized pore sizes for large biomolecules
- High target recovery – for improved process economics
- High mechanical stability – for HTP purification
- Optimal pore size – for efficient large-biomolecule movement
- Ideal particle size and range – to maintain high DBC and reproducibility
- Fast mass transfer kinetics – to increase process efficiency
- Broad chemical stability and compatibility – for ease of use
Screen this resin for your application. Request a sample.
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions.

