CHT XT Media is the newest addition to Bio-Rad’s ceramic hydroxyapatite line of resins, which are touted as the industry gold standards for aggregate removal. It is a robust, long-lasting media for repeated use and exceptional resolution. It combines the functionality of CHT Type I with a long column life to help achieve high productivity and improved process economics.
Achieve exceptional resolution of proteins across a wide range of pIs
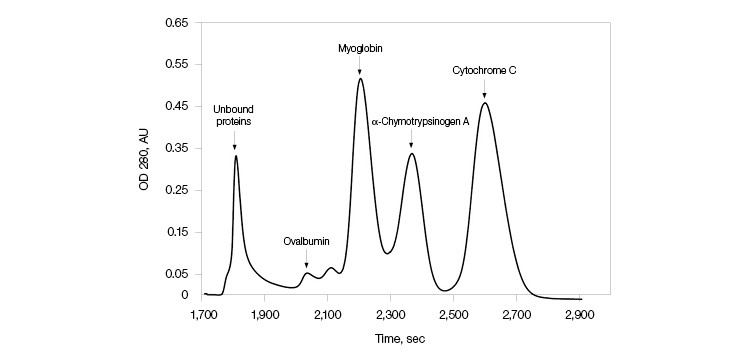
A protein mixture with four different proteins ranging from pI of 4.5 to 9.5 was used to demonstrate the resolution properties of CHT XT. Figure 1 shows efficient separation of the mixture.
| Flow rate: | 1.57 ml/min |
| Pre-equilibration: | 400 mM sodium phosphate, pH 6.8, 4 CV |
| Loading buffer: | 5 mM sodium phosphate, pH 6.8, 15 CV |
| Post-load wash: | 5 mM sodium phosphate, pH 6.8, 1 CV |
| Elution: | Linear gradient elution 0-75% 400 mM sodium phosphate, pH 6.8, over 15 CV |
| Stripping: | 400 mM sodium phosphate, pH 6.8, 3 CV |
| Sanitization: | 1 M sodium hydroxide, 3 CV |
Fig. 1. Separation of protein standards. A mixture of the following protein samples in 10 ml of 5 mM sodium phosphate, pH 6.8, was used for purification: 120 mg of ovalbumin, 75 mg of myoglobin, 60 mg of α-chymotrypsinogen A, 75 mg of cytochrome C. The mixture (100 µl) was loaded onto a 0.7 x 5.4 cm column with a packed bed volume of 2 ml of CHT XT.
Accomplish single-step clearance of aggregates and other impurities
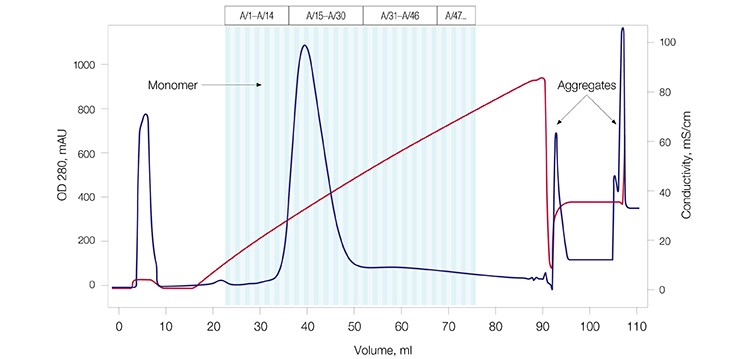
A monoclonal antibody sample (mAb G) was used to test the aggregate removal and antibody purification capability of CHT XT. The mAb G sample contained a significant amount (~28%) of aggregate as shown by size exclusion chromatography (SEC) analysis (Figure 2A). This sample was purified using CHT XT (Figure 2B) and the eluate from the resultant peak was again analyzed by SEC (Figure 2C). Complete separation of the mAb aggregates from the monomers was observed, demonstrating that CHT XT is highly effective at aggregate removal during monoclonal antibody purification.
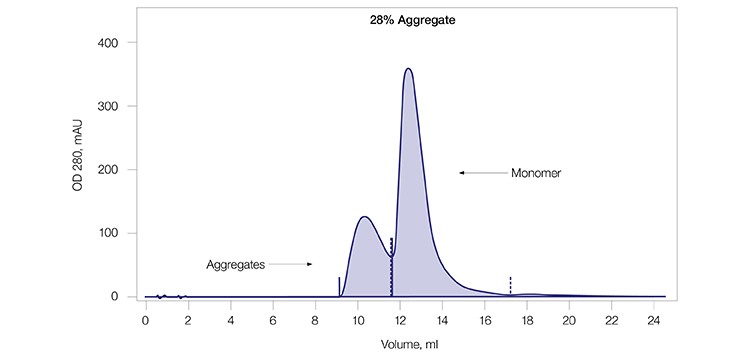
Fig. 2A. SEC of mAb G load. SEC showing the level of mAb G aggregates in the load. mAb G was loaded on a 10 x 300 mm Bio-Rad ENrich SEC 650 Column with a packed bed volume of 23.56 ml equilibrated in PBS.
| Sample load: | 40 mg mAb G sample with aggregates |
| Column: | 1.85 ml CHT XT packed in a 0.5 x 9.4 cm column |
| Flow rate: | 1 ml/min |
| Equilibration: | 10 mM sodium phosphate, pH 6.5, 10 CV |
| Wash: | 10 mM sodium phosphate, pH 6.5, 3 CV |
| Elution: | Linear gradient elution 0-100% 10 mM sodium phosphate, 1 M sodium chloride, pH 6.5 |
| Fraction collection: | 1 ml fraction size over 40 CV |
| Stripping: | 0.4 M sodium phosphate, pH 7, 4 CV |
| Sanitization: | 1 M sodium hydroxide, 3 CV |
Fig. 2B. mAb G purification profile. Elution profile showing separation of the monomer from higher molecular weight impurities. OD 280 (—); conductivity (—).
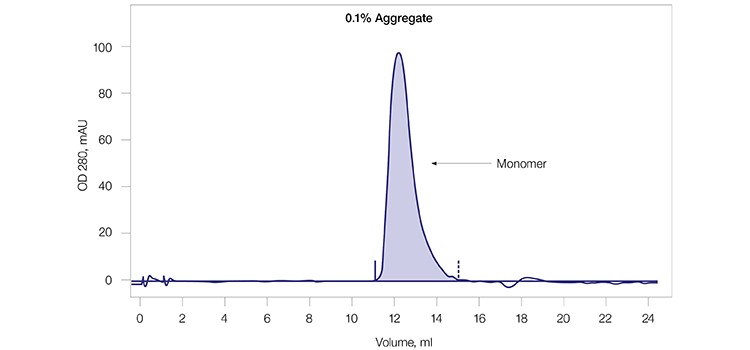
Fig. 2C. Final SEC profile of mAb G monomer pool. The SEC profile of the pooled fractions from CV 13–27 (A/13–A/27 in Figure 2B) confirms aggregate clearance from the mAb G monomer.
Attain repeated reusability and increase cost efficiency with the long life time of CHT XT
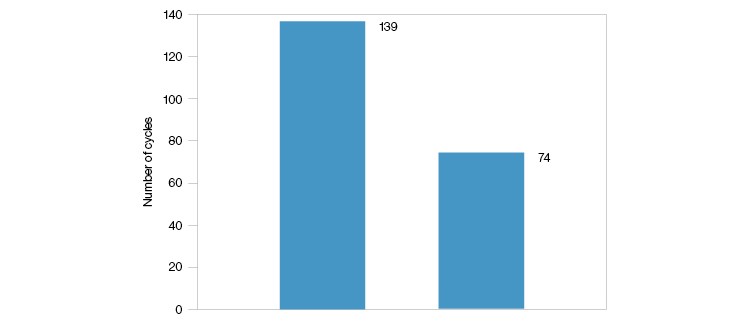
CHT XT can be used for over 70 cycles (Figure 3) under the listed conditions, making it ideal for manufacturing processes.
| Protocol I | |
| Pre-equilibration: | 0.4 mM sodium phosphate, pH 7.0, 4 column volumes (CV) |
| Equilibration, Loading and wash: | 5 mM sodium phosphate, 0.1 M sodium chloride, pH 6.5, 15 CV |
| Elution: | 5 mM sodium phosphate, 0.55 M sodium chloride, pH 6.5, 4 CV |
| Stripping: | 0.4 M sodium phosphate, pH 7, 3 CV |
| Sanitization: | 1 M sodium hydroxide, 3 CV |
| Protocol II | |
| Pre-equilibration: | 0.4 mM sodium phosphate, pH 6.5, 4 CV |
| Equilibration, Loading and wash: | 5 mM sodium phosphate, pH 6.5, 15 CV |
| Elution: | 5 mM sodium phosphate, 0.55 M sodium chloride, pH 6.5, 4 CV |
| Stripping: | 0.4 M sodium phosphate, pH 6.5, 3 CV |
| Sanitization: | 1 M sodium hydroxide, 1 M sodium chloride, 3 CV |
Fig. 3. Column lifecycle study of CHT XT. A 20 x 20 cm column was packed with CHT XT and cycled continuously at 140 cm/hr using buffer protocols I or II until a column backpressure of 3 bar was reached.
In summary, CHT XT provides the following benefits for process purification:
- Unique selectivity
- Efficient single-step clearance of aggregates and other impurities
- Straightforward column packing
It comes with full regulatory support and column packing and method development services.
Screen this resin for your application. Request a sample.
Bio-Rad and CHT are trademarks of Bio-Rad Laboratories, Inc. in certain jurisdictions.

