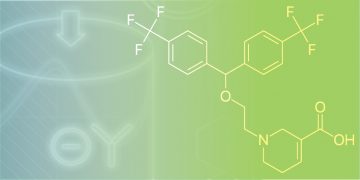
Single-Step Influenza Virus Purification Using an Anion Exchange Resin

Powering Antibody Production and Purification with the NGC Chromatography System

The Process of Resin Screening

Bioprocessing Recombinant Proteins

Purification of Untagged Proteins Made Attainable for Any Researcher

Automate Your Antibody Purification Workflow at Preparative and Analytical Scale
Presented by: Katie Schaefer, PhD, Global Product Manager, Bio-Rad
Peter Winship, PhD, Technical Product Manager, Teledyne Cetac Technologies
View on demand
Purification is a multistep process toward confirmation of a target protein’s purity. In this webinar you will learn how to automate your protein purification workflow by following the creation of a hypothetical monoclonal antibody (mAb). Dr. Schaefer and Dr. Winship expertly demonstrate how the NGC Chromatography System and the AEX-500 series Autosampler work together to maximize screening capability as well as rapidly achieve therapeutic development objectives.

Are biofuels good for the planet?
Serving your Downstream Purification Needs
How to Make a Successful Vaccine