Introduction
Many essential functions in living cells are mediated by cell membranes, which are made up primarily of a lipid bilayer with proteins embedded in them. The genes coding for membrane proteins, such as G-protein coupled receptors (GPCR), ion channels, and other membrane-bound enzymes make up 25–30% of the human genome and membrane proteins are estimated to represent 30–40% of the top drug targets. There is growing research, industrial, and commercial interest in the study of membrane proteins in general, and more specifically in immobilizing membrane proteins to various biosensor surfaces (Fruh et al. 2011).
Surface plasmon resonance (SPR) is a direct label-free technique that provides real-time data on the affinity, specificity, and kinetics of biomolecular interactions. SPR-based biosensors are among the most sensitive and accurate optical biosensors available today. Yet most SPR studies describe only soluble protein-protein interactions and the number of publications describing membrane proteins studied with SPR is limited (Rich and Myszka, 2009). Membrane proteins are typically analyzed in biosensors after incorporation into liposomes or lipoparticles (virus-like particles). However, biosensor experiments are often hindered by the inefficient capture of these liposomes/lipoparticles to the biosensor surface.
The ProteOn XPR36 protein interaction array system and One-shot Kinetics™ approach expand the capabilities of the traditional SPR workflow by enabling multiple quantitative binding experiments to be run in parallel. The ProteOn system integrates a unique 6 x 6 interaction array for the analysis of up to six ligands with up to six analytes to produce 36 data points simultaneously. The multiplexing capability of this system enables capture of the ligand (or liposomes, in the case of membrane proteins) using several methods, and then comparison of the complete kinetic profiles of the interactions.
The memLAYER products used in this study are based on the proprietary DNA tether enhanced liposome immobilization (TELI) technology from Layerlab AB (Göteborg, Sweden). The memLAYER technology can be used in various applications, including the transport of molecules over cell membrane that is mediated via membrane proteins such as aquaporins and melittin, activity of membrane proteins such as GPCR and clotting factors, and passive diffusion of small uncharged molecules over liposome membrane (Branden et al. 2008; Branden et al. 2010). Further details on this technology can be found at www.layerlab.se.
In this report we describe the use of the ProteOn XPR36 biosensor array, together with two complementary techniques, for the kinetic analysis of membrane proteins and potential therapeutic antibodies. A novel efficient capture method, the memLAYER chemistry kit from Layerlab, was used for enhanced capture of liposomes and GPCR-containing lipoparticles obtained from Integral Molecular. The kinetic results of a specific anti-GPCR antibody were compared for the following different capturing methods: (1) immobilization of lipoparticles by amine coupling; (2) capturing lipoparticles through antibody directed against the membrane protein CD81, a cell surface transmembrane glycoprotein expressed by the lipoparticle producing cells, which allows capturing the lipoparticles by a monoclonal anti-CD81 antibody; (3) capturing lipoparticles on immobilized undecylamine, a long lipophilic molecule with an amine end, which can be covalently bound to the carbohydrate matrix of the GLM chip; and (4) capturing lipoparticles through DNA tethers using Layerlab technology.
Materials and Methods
Instrumentation and Reagents
Experiments were performed using the ProteOn XPR36 system with ProteOn GLM and NLC sensor chips. The running buffer for liposome and lipoparticle immobilization was ProteOn PBS (phosphate buffered saline, pH 7.4). All experiments were performed at 25°C. The ProteOn amine coupling reagents used were EDAC (N-ethyl-N’-(3-dimethylaminopropyl) carbodiimide), sulfo-NHS (N-hydroxysuccinimide), and 1 M ethanolamine hydrochloride solution, pH 8.5. memLAYER chemistry kit and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) liposomes were from Layerlab AB. Lipoparticles containing the GPCR CXCR4 were from Integral Molecular. NeutrAvidin was from Pierce. Anti-human CD81 and anti-CXCR4 were from BD Pharmingen.
Liposome Capture
Single and double layers of liposomes were captured on an NLC chip using the memLAYER chemistry kit (Figure 1). First, POPC liposomes (4 mg/ml) and cholesterol-modified DNA (0.4 µM chol-DNA1 tag) were incubated for 30 min at room temperature to yield chol-DNA1 tagged liposomes. Two channels of an NLC sensor chip were modified with 50 µl of biotin-DNA2 (2 µM) at a flow rate of 25 µl/min. Next, 250 µl of chol-DNA1 tagged liposomes (2 mg/ml) were injected into channel 1, and 250 µl of nontagged control liposomes (4 mg/ml) were injected into the second channel. To create the double layer capture, a second DNA injection of 50 µl of chol-dsDNA2 (0.4 µM) at 25 µl/min was done, followed by 250 µl of the chol-DNA1 tagged liposomes (2 mg/ml). The surface was regenerated using a single injection of 150 µl DDW causing dehybridization of the dsDNA and release of the liposomes.
Lipoparticles
The lipoparticles were produced by Integral Molecular (Philadelphia, PA, U.S.). They are retroviral virus-like particles (VLP) containing specific cellular membrane proteins for use as biochemical tools to study GPCRs, ion channels, and other membrane proteins. Lipoparticles are a convenient and consistent source of membrane proteins and maintain membrane proteins in their native lipid environment. Because of their small size (~150 nm) and purity, lipoparticles can be adapted to instruments and formats, such as microfluidics, microarrays, beads, and biosensors, that are otherwise difficult for membrane proteins. Lipoparticles typically contain 50–200 pmol/mg of overexpressed receptor, which translates into approximately 50–200 receptors per lipoparticle (Willis et al. 2008) In addition to the specific receptor, lipoparticles contain additional membrane proteins from the cells used for production, and those proteins could be utilized for capturing the lipoparticles using amine coupling or antibody capturing. Further details on this technology can be found at www.integralmolecular.com.
Lipoparticle Capture
Lipoparticles containing CXCR4 (chemokine receptor type 4) were immobilized on a GLM chip using four different methods. All immobilization and capturing was done with PBS as the running buffer, at a flow rate of 25 µl/min.
Amine coupling immobilization of lipoparticles — one vertical channel of a GLM chip was activated using 100 µl of 40 mM EDAC, 10 mM s-NHS. The CXCR4 lipoparticles were diluted into sodium acetate, pH 4.0 to concentration of 100 µg/ml, and 180 µl were injected onto the activated channel for immobilization. The channel was then deactivated with an injection of 180 µl of 1 M ethanolamine-HCl, pH 8.5.
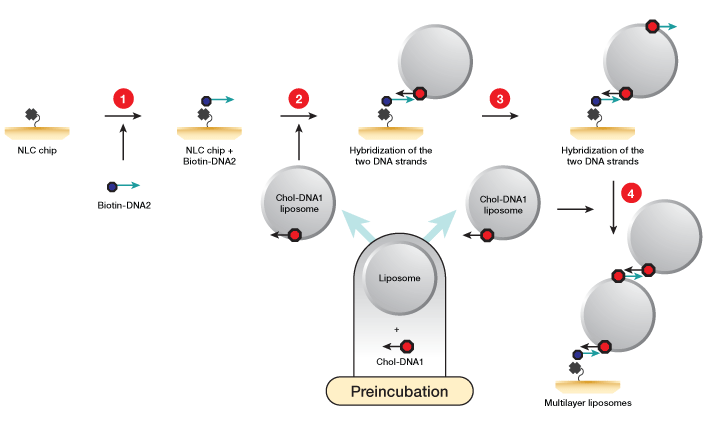
Fig. 1. Workflow for liposome capture using the memLAYER kit. DNA1 and DNA2 symbolize complementary sequences. 1, the NLC chip NeutrAvidin surface is saturated with single-stranded biotinylated DNA2 molecules; 2, liposomes are incubated with cholesterol-DNA1 to create liposomes loaded with DNA complementary to the biotin DNA at the sensor surface. These liposomes are then injected over the chip surface, generating a monolayer of liposomes; 3, cholesterol-DNA2 is injected over the liposomes; 4, a second injection of the cholesterol-DNA1 liposomes yields the double layer.
2. Capturing lipoparticles by CD81 antibody — the second vertical channel of the GLM chip was activated using 100 µl of 40 mM EDAC, 10 mM S-NHS. Anti-human CD81 was diluted in sodium acetate, pH 4.5, to concentration of 25 µg/ml, and 180 µl were injected onto the activated channel for immobilization. The channel was then deactivated with an injection of 180 µl of 1 M ethanolamine-HCl, pH 8.5. Finally, 425 µl of CXCR4 lipoparticles (70 µg/ml in PBS) were injected for capturing by the anti-human CD81.
3. Capturing lipoparticles by undecylamine — the third vertical channel of the GLM chip was activated using 50 µl of 40 mM EDAC, 10 mM S-NHS. Undecylamine was diluted in sodium acetate, pH 5.0, with 5% DMSO to concentration of 2.3 mM, and 180 µl were injected onto the activated channel for immobilization. The channel was then deactivated with an injection of 180 µl of 1 M ethanolamine-HCl, pH 8.5, followed by a 50 µl injection of 50 mM NaOH. Finally, 425 µl of CXCR4 lipoparticles (70 µg/ml in PBS) were injected for capturing by the undecylamine.
4. Capturing of lipoparticles using memLAYER method; Single and double layers — two vertical channels of a GLM chip were activated using 100 µl of 40 mM EDAC, 10 mM S-NHS. NeutrAvidin (NA) was diluted in sodium acetate pH 4.5 to concentration of 50 µg/ml, and 180 µl were injected onto the activated channels for immobilization. The channels were then deactivated with an injection of 180 µl of 1 M ethanolamine-HCl, pH 8.5. Biotin-DNA2 at a concentration of 1 µM (50 µl) in PBS was injected followed by an injection of 250 µl of 0.3 mM of cholesterol-dsDNA1. Then 425 µl of CXCR4 lipoparticles (70 µg/ml in PBS) were injected to the channel.
To form the second layer of lipoparticles, 250 µl of chol-ds DNA2 0.3 µM was injected followed by 250 µl of 0.3 µM cholesterol-dsDNA1. Finally, a second injection of 425 µl of CXCR4 lipoparticles (70 µg/ml in PBS) was performed (Figure 2).
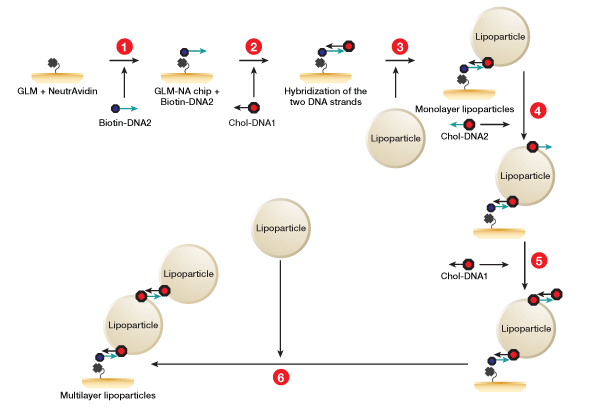
Fig. 2. Workflow for lipoparticle capture using the memLAYER kit. DNA1 and DNA2 symbolize complementary sequences. 1, NeutrAvidin is amine-coupled on GLM chip surface, and then saturated with single stranded biotinylated DNA2 molecules; 2, cholesterol-DNA1 is injected and hybridizes with the surface DNA2; 3, the CXCR4 lipoparticles are captured on the cholesterol surface, generating a monolayer of lipoparticles; 4, cholesterol-DNA2 is injected over the lipoparticles; 5, cholesterol-DNA1 is injected and hybridizes with the lipoparticles DNA2; 6, a second injection of the lipoparticles on the cholesterol surface yields the double layer.
Analyte injection — CXCR4 antibody
Anti-CXCR4 analyte sample was prepared by serial dilution in PBS to final concentrations of 30, 15, 7.5, 3.75, and 1.875 nM. The five analyte concentrations were injected (150 µl) orthogonal to the ligand channels at a flow rate of 100 µl/min. Running buffer was injected into the sixth channel for real-time double referencing.
Kinetic Analysis
The data were analyzed with the ProteOn Manager™ 2.1 software. Binding curves were processed for baseline and injection start alignment, together with channel reference subtraction using an empty channel. Each set of five reference-subtracted sensorgrams was fitted globally to curves describing a homogeneous 1:1 bimolecular reaction model. Each CXCR4 lipoparticle ligand surface interacting with its analyte concentration was grouped together to fit the ka, kd and Rmax parameters, and the equilibrium dissociation constant, KD, was calculated using the equation KD = kd/ka.
Results and Discussion
Highly Efficient Capture of Two Layers of Liposomes Using memLAYER Technology
Single or double layers of liposomes were successfully captured on a ProteOn chip using Layerlab capture technology (Figure 3). Two channels of an NLC chip surface were saturated with biotinylated single-stranded DNA2, and liposomes carrying the complementary cholesterol-DNA1 tag were then bound to the surface through hybridization, reaching a saturation density of 8,140 RU. To examine the nonspecific binding of the liposomes to the chip surface, nontagged naked liposomes were simultaneously injected on the second DNA2-modified channel, resulting in 540 RU binding. This low binding of the naked liposomes to the surface indicates that most of the DNA-tagged liposomes are bound to the surface in a specific manner through the DNA tags.
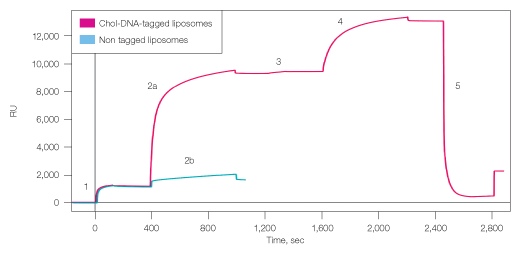
Fig. 3. Capture of single and double layers of liposomes to ProteOn NLC sensor chip. 1, binding biotin-DNA2 to chip surface; 2a, capturing cholesterol-DNA1 tagged liposomes; 2b, capturing nontagged liposomes (control); 3, incorporating cholesterol-dsDNA2 into the bound liposomes; 4, capturing second layer of cholesterol-DNA1 tagged liposomes; 5, regeneration with DDW. RU=response units.
To create the second liposome layer, cholesterol-DNA2 was incorporated into the surface-bound liposomes, and liposomes carrying the complementary DNA1 tag were injected on top. The second layer added density of 3,600 RU, giving total density of almost 12,000 RU liposomes. Regeneration with DDW efficiently removed 90% of the liposome layers, leaving ~1,000 RU of liposomes. This residual density could result from nonspecifically bound liposome or liposome fragments on the chip surface.
Parallel Immobilization of CXCR4 Lipoparticles Using Different Techniques
The CXCR4 chemokine receptor is a 7-transmembrane GPCR expressed in immune system cells and various cancer cells. In addition, CXCR4 acts together with CD4 as a co-entry receptor to HIV. The clinical importance of CXCR4 has made it a major drug target, and many CXCR4-blocking antibodies showing anti-HIV, anti-inflammatory, and/or anti-cancer activities are under preclinical evaluation and development. Here, we used CXCR4 and its monoclonal antibody 12G5, as a model for kinetic analysis of membrane proteins in their natural environment — the cell membrane. For this purpose, CXCR4 lipoparticles were captured using different approaches: direct amine coupling or capturing through antibodies, lipophilic chains, and DNA tethers.
The immobilization of lipoparticles by amine coupling is possible since their membrane is embedded with the overexpressed receptor, as well as with other native membrane proteins from the original producing cells. Here, the CXCR4 lipoparticles were diluted in acetate buffer, pH 4.0, to allow their electrostatic attraction to the negatively charged surface of the activated GLM chip and the subsequent covalent binding. Using this approach, average density of 1,850 relative units (RU) was obtained for the CXCR4 lipoparticles on the six interaction spots of the channel (Figure 4).
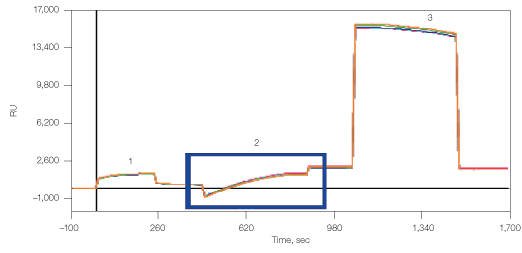
Fig. 4. Immobilization of lipoparticles by amine coupling. 1, chip surface activation with EDAC/S-NHS; 2, binding of CXCR4 lipoparticles; 3, deactivation with ethanolamine.
Amine coupling of the anti-CD81 to the GLM chip resulted in 6,840 RU density, which thereafter captured 1,800 RU lipoparticles (Figure 5). The lipoparticle capture in this case did not reach saturation and, if desired, higher density of the lipoparticles could have been obtained.
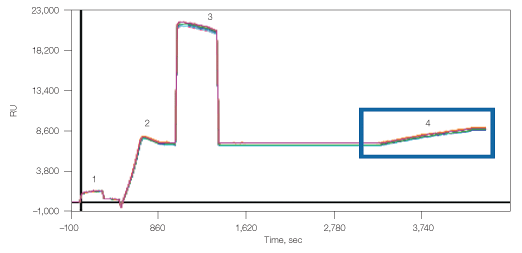
Fig. 5. Capture of lipoparticles by CD81 antibody. 1, chip surface activation with EDAC/S-NHS; 2, binding of anti-human CD81; 3, deactivation with ethanolamine; 4, capturing of CXCR4 lipoparticles.
The third binding approach used was through undecylamine, which can be covalently bound to the carbohydrate matrix of the GLM chip (Figure 6). This surface can then be used for capturing liposomes and membrane preparations from cell lysates. Here, amine coupling of the undecylamine resulted in 660 RU, which allowed the capturing of 4,000 RU lipoparticles.
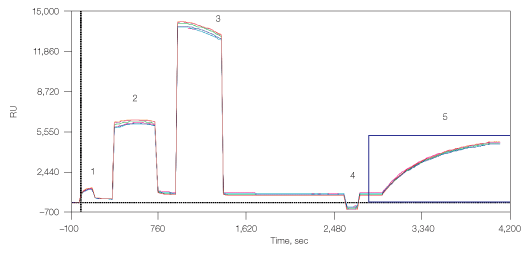
Fig. 6. Capturing lipoparticles by undecylamine. 1, chip surface activation with EDAC/S-NHS; 2, binding of undecylamine; 3, deactivation with ethanolamine; 4, stabilization by NaOH 50 mM; 5, capturing of CXCR4 lipoparticles.
Finally, the CXCR4 lipoparticles were also captured using the memLAYER technology in a manner similar to the liposome capturing described above (Figure 7). This resulted in 2,500 RU density of the first lipoparticle layer and additional 1,000 RU density of the second lipoparticle layer.
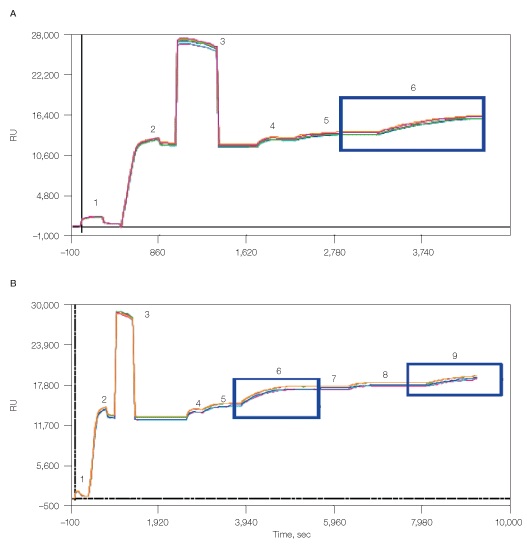
Fig. 7. Capturing of lipoparticles using memLAYER method, single layer (A) and double layer (B). 1, chip surface activation with EDAC/S-NHS; 2, binding of NeutrAvidin; 3, deactivation with ethanolamine; 4, biotin-DNA2 injection; 5, cholesterol-DNA1 hybridization; 6, capturing of CXCR4 lipoparticles; 7, cholesterol-DNA2 injection; 8, cholesterol-DNA1 injection; 9, capturing of CXCR4 lipoparticles.
Determination of Anti-CXCR4 Kinetic Constants in Native Membrane Environment
The kinetic rate constants of membranal CXCR4 binding by its antibody were determined using the ProteOn One-shot Kinetics approach (Figure 8). In a single injection, five antibody concentrations were injected over the different lipoparticle surfaces, allowing the simultaneous measure of full kinetic profiles for all capturing methods, with no need for regenerations between the different concentrations. In addition, the same injection also included a blank sample (running buffer with no analyte), which allowed for real-time double referencing. For each capturing method, the slow dissociation of the captured lipoparticles is measured at the time of the interaction itself, allowing for accurate and reliable double referencing without the need to perform a separate blank injection.
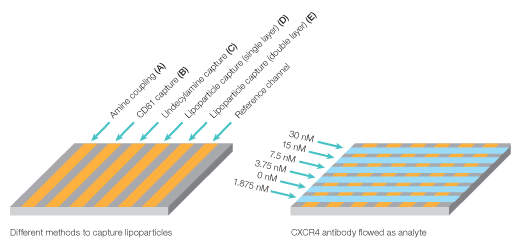
Fig. 8. Experiment workflow for multiplexed analysis of CXCR4 with its antibody.
The binding sensorgrams with the overlaid 1:1 Langmuir binding model fit are shown in Figure 9, and the kinetic binding constants are summarized in Table 1. In all cases, the data fit well wth the 1:1 model, and using a bivalent analyte model did not improve the fit. This indicates that the CXCR4 proteins are dispersed in the lipoparticle membranes such that each antibody can bind only a single CXCR4 molecule. Very similar binding rate constants were obtained for the CXCR4-antibody interaction when the lipoparticles were captured by different methods, with an average KD of 0.05 nM.
The accessibility to antibody binding and integrity of the membrane CXCR4 protein was compared by calculating the ratio between the analyte Rmax and the lipoparticles’ density in each of the immobilization methods (Table 1). It appears that the highest activity was obtained using the single layer of memLAYER bound lipoparticles, but adding a second layer using this technology reduced the activity of the bound proteins. Binding the lipoparticles by undecylamine, amine coupling, and anti-CD81 resulted in even lower activity. In these methods, the lipoparticles are probably attached very close to the chip’s surface. By binding through the memLAYER DNA tethers, the lipoparticles and the membranal proteins are located away from the surface, allowing higher accessibility to the antibody binding.
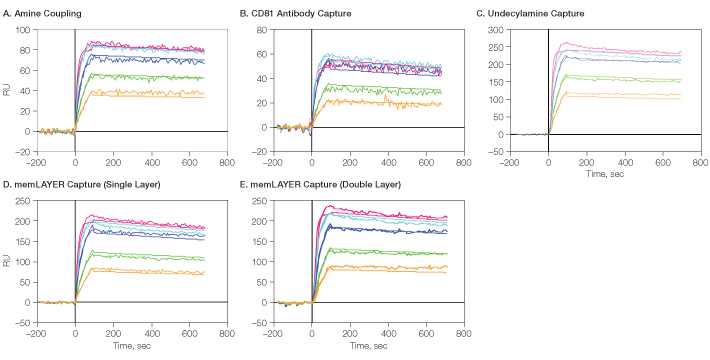
Fig. 9. Detailed kinetic analysis of CXCR4 membrane protein with its antibody. CXCR4 lipoparticles were immobilized on GLM using the four described methods, followed by One-shot Kinetics injection of CXCR4 antibody. A through E show the sensorgrams for the five analyte concentrations with the overlaid 1:1 model fit. RU, relative units.  , 30 nM;
, 30 nM;  , 15 nM;
, 15 nM;  , 7.5 nM;
, 7.5 nM;  , 3.75 nM;
, 3.75 nM;  , 1.875 nM
, 1.875 nM
Table 1. Kinetic analysis of CXCR4 membrane protein with its antibody.
| Method | Density, RU |
ka, 1/Ms |
kd, 1/s |
KD, M |
Rmax, RU |
Activity, Rmax/density |
| Amine coupling | 1,850 | 3.26 × 106 | 1.12 × 10–4 | 3.4 × 10–11 | 84 | 0.045 |
| Via CD81 | 1,800 | 3.00 × 106 | 2.24 × 10–4 | 7.5 × 10–11 | 56 | 0.031 |
| Undecylamine | 4,000 | 3.65 × 106 | 1.25 × 10–4 | 3.4 × 10–11 | 244 | 0.061 |
| memLAYER single layer | 2,500 | 2.82 × 106 | 1.90 × 10–4 | 6.7 × 10–11 | 203 | 0.081 |
| memLAYER double layer | Total 3,500 | 2.77 × 106 | 1.54 × 10–4 | 5.6 × 10–11 | 222 | 0.063 |
To conclude, all these experiments demonstrate the high throughput and efficient assay optimization capabilities of the ProteOn interaction array system. On a single chip, five different immobilization methods for lipoparticles have been shown, and the full kinetic profile of an antibody directed against a membrane GPCR protein was obtained in a single injection. All techniques tested gave sufficient lipoparticle immobilization levels, and the calculated kinetic constants were similar. It was demonstrated that lipoparticles captured using the memLAYER technology are more active and accessible than those captured by other methods.
References
Branden et al. (2008). Label-free measurements of molecular transport across liposome membranes using evanescent-wave sensing. Chemphyschem 9, 2480–2485.
Branden et al. (2010). Refractive-index-based screening of membrane-protein-mediated transfer across biological membranes. Biophys J 99, 124–133.
Fruh et al. (2011). How to catch a membrane protein in action: a review of functional membrane protein immobilization strategies and their applications. Chem Rev 111, 640–656.
Rich and Myszka (2010). Grading the commercial optical biosensor literature — class of 2008: ‘The Mighty Binders’. J Mol Recognit 23, 1–64.
Willis et al. (2008). Virus-like particles as quantitative probes of membrane protein interactions. Biochemistry 47, 6988–6990.
Legal Notice
memLAYER is a trademark of Layerlab AB. Integral Molecular is a trademark of Integral Molecular, Inc. NeutrAvidin is a trademark of Pierce Biotechology, Inc.

