
Bio-Rad’s new CFX qualification plate accurately verifies the performance of any CFX real-time PCR system, giving you confidence in your results. Preloaded with an optimized mastermix containing Bio-Rad’s high-performance SsoAdvanced™ SYBR® Green supermix, the CFX qualification plate provides a five-point standard curve, two unknown groups, and no template controls (NTCs) for quickly determining a pass/fail instrument performance result.
The CFX qualification plate offers:
- 3-step run start with a predispensed plate and predefined thermal cycling protocol and plate templates
- 1-click pass/fail performance result with CFX Manager™ software
- 2-fold discrimination with a 99.7% confidence level and a single product melt peak criterion for determining pass/fail results
- Easy incorporation into an instrument qualification procedure with a printable report
Included in Bio-Rad’s user-friendly CFX Manager software is the new Qualification Plate Check tool for analyzing the CFX qualification plate data. The Qualification Plate Check tool uses an algorithm to determine a pass/fail performance result based on two criteria, a twofold discrimination with 99.7% confidence level and the single product melt peaks for the two unknowns (Figure 1.). A passing result indicates good optics performance and uniform thermal electric (TE) control in the thermal block. By examining the two unknown groups and their melt peaks, the Qualification Plate Check tool can reliably determine the performance status of any CFX real-time PCR system.
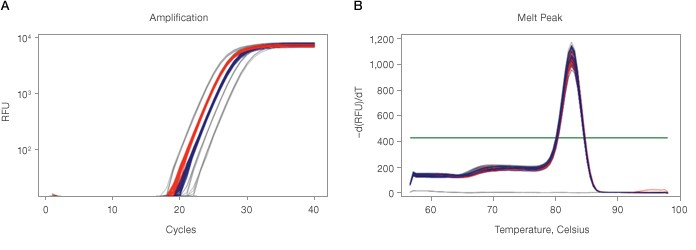
Fig. 1. Amplification (A) and melt peak (B) charts demonstrating good instrument performance in a CFX96 Touch™ real-time PCR detection system. RFU, relative fluorescence units.
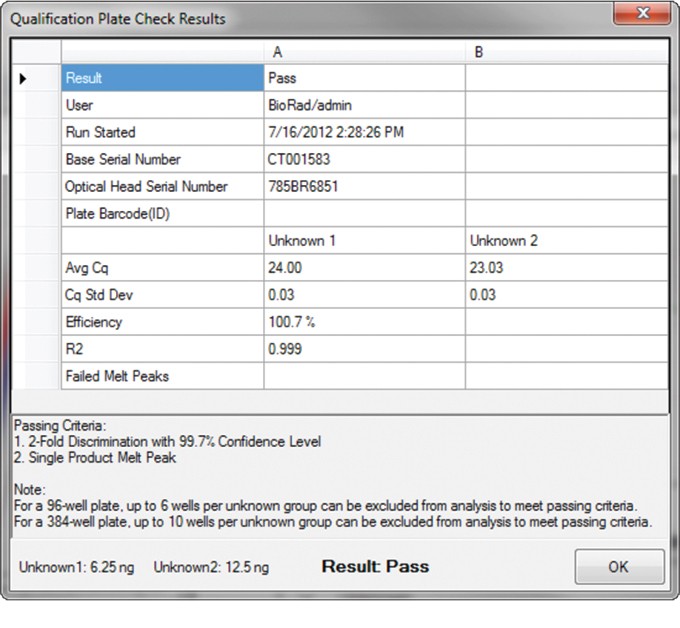
Fig. 2. Qualification Plate Check Results window displays passing results for a CFX96 Touch system.
The Qualification Plate Check tool produces a Qualification Plate Check Results window displaying a pass/fail instrument performance result and other details, such as base serial number, optical head serial number, and plate bar code ID for easy traceability of system verification (Figure 2). The Qualification Plate Check Results window can be printed or copied for easy inclusion into an instrument qualification protocol.
Ordering Information
| Catalog# | Description |
| 1845098 | CFX Qualification Plate, 96-well format, includes 96-well plate containing template DNA, primers, SsoAdvanced™ SYBR® Green supermix, and nuclease-free water, and instruction manual |
| 1845099 | CFX Qualification Plate, 384-well format, includes 384-well plate containing template DNA, primers, SsoAdvanced™ SYBR® Green supermix, and nuclease-free water, and instruction manual |
Bio-Rad’s real-time thermal cyclers are licensed real-time thermal cyclers under Applera’s U.S. Patent Number 6,814,934 B1 for use in research, human in vitro diagnostics, and all other fields except veterinary diagnostics.
CFX real-time PCR detection systems are covered by one or more of the following U.S. patents or their foreign counterparts owned by Eppendorf AG: U.S. Patent Numbers 6,767,512 and 7,074,367.
SYBR is a trademark of Life Technologies Corp. Bio-Rad Laboratories, Inc. is licensed by Life Technologies Corporation to sell reagents containing SYBR Green I for use in real-time PCR, for research purposes only.

