The current gold standard in monoclonal antibody purification for most researchers is affinity purification with Protein A or G followed by size exclusion chromatography (SEC). Although robust and highly effective, this workflow does have critical pain points and inefficiencies, leaving researchers to feel that they must oversee every step of their workflow, not leaving their chromatography system unattended for more than a few minutes.
Bottlenecks and inefficiencies in the antibody purification workflow are introduced with the requisite low pH elution step. While required for eluting bound antibodies from the column, extended exposure to low pH promotes antibody aggregation and decreases overall monodispersed yield (Mazzer et al. 2015), necessitating pH neutralization and aggregate removal downstream. The cumulative hands-on processing and manual intervention required increases the risk for inconsistencies in antibody purity and yield, both day-to-day and among operators. When processing multiple antibodies, researchers feel like they are working for the chromatography machine more than it is working for them. A hands-off solution will free up researchers’ time, remove frustration, and improve antibody yield and consistency.
Automating Monoclonal Antibody Purification
The NGC Chromatography System was developed to streamline monoclonal antibody purification with a tandem workflow that addresses the most common inefficiencies associated with traditional purification methods. The comprehensive solution, which includes Protein A affinity purification, automated in-line antibody neutralization, and SEC facilitates walk-away, reproducible production of high-quality antibodies at the tens-of-milligrams scale. With valves to empower automation and user-friendly ChromLab Software for customizable system and method programming, the NGC Chromatography System is both accessible and adaptable for all users, from novice to expert.

The NGC Chromatography System is highly customizable to fit your purification needs.
A Customized, Worry-Free Solution
The benefits and ease of use of this system were demonstrated with a custom NGC Quest 100 Plus Chromatography System, configured for optimal purification efficiency of the biologic drug trastuzumab (Hilario et al 2022; Figure 1). Automated, two-column tandem purification with Protein A affinity purification, SEC, and intermediate in-line neutralization was facilitated by the addition of several additional valves and accessories to the base system.
Two column switching valves (CSVs) allow the columns to be programmed in and out of the flow path as needed while continuously monitoring their respective delta-column pressures. The first CSV is plumbed with a 5-ml EconoFit UNOsphere SUPrA Protein A Column and the second CSV is plumbed with a 120-ml Hi-Prep 16/60 Sephracryl S-300 HR SEC Column. Elution of the UNOSphere SUPrA Column in reverse flow helps minimize elution volume, which should not exceed 5 ml, and enables appropriate resolution without the need for intermediate concentration steps or multiple SEC runs.
Samples (500 ml trastuzumab-containing supernatant) are loaded directly onto the Protein A column with the NGC sample pump. Traditionally, system pump A and system pump B work together, feeding into the mixer for precise linear gradient formation. In this custom system, the NGC System plumbing is configured to drive the primary purification isostatically via system pump A, which is fitted with an upstream inlet valve that enables switching between the primary running buffer (PBS, pH 7.8) and the Protein A elution buffer (0.1 M glycine, pH 3.0).
System pump B is designated for delivering a pH neutralizing solution simultaneously with IgG elution from the UNOSphere SUPrA Column prior to loading onto the SEC column. To deliver the neutralization buffer (1 M Tris, pH 8.0), the pump is connected into the flow path between the column switching valve with a static Y-mixer chamber. The eluate (0.1 M glycine, pH 2.9) and neutralization buffer mixture are then directed to the SEC column on CSV 2. Large insoluble materials are removed by a 5 µm in-line filter placed after CSV 1 and before the Y-mixer.
A pH detector in conjunction with the Multiwavelength Detector allows for simultaneous real-time monitoring of up to four wavelengths of choice from 190–800 nm, conductivity, and pH. Ultra-pure IgG samples are collected using the NGC Fraction Collector. The potential upper limit of antibody yield is determined by the dynamic binding capacity of UNOSphere SUPrA Resin; for example, the binding capacity of a 5 ml column is 150 mg based on human IgG.
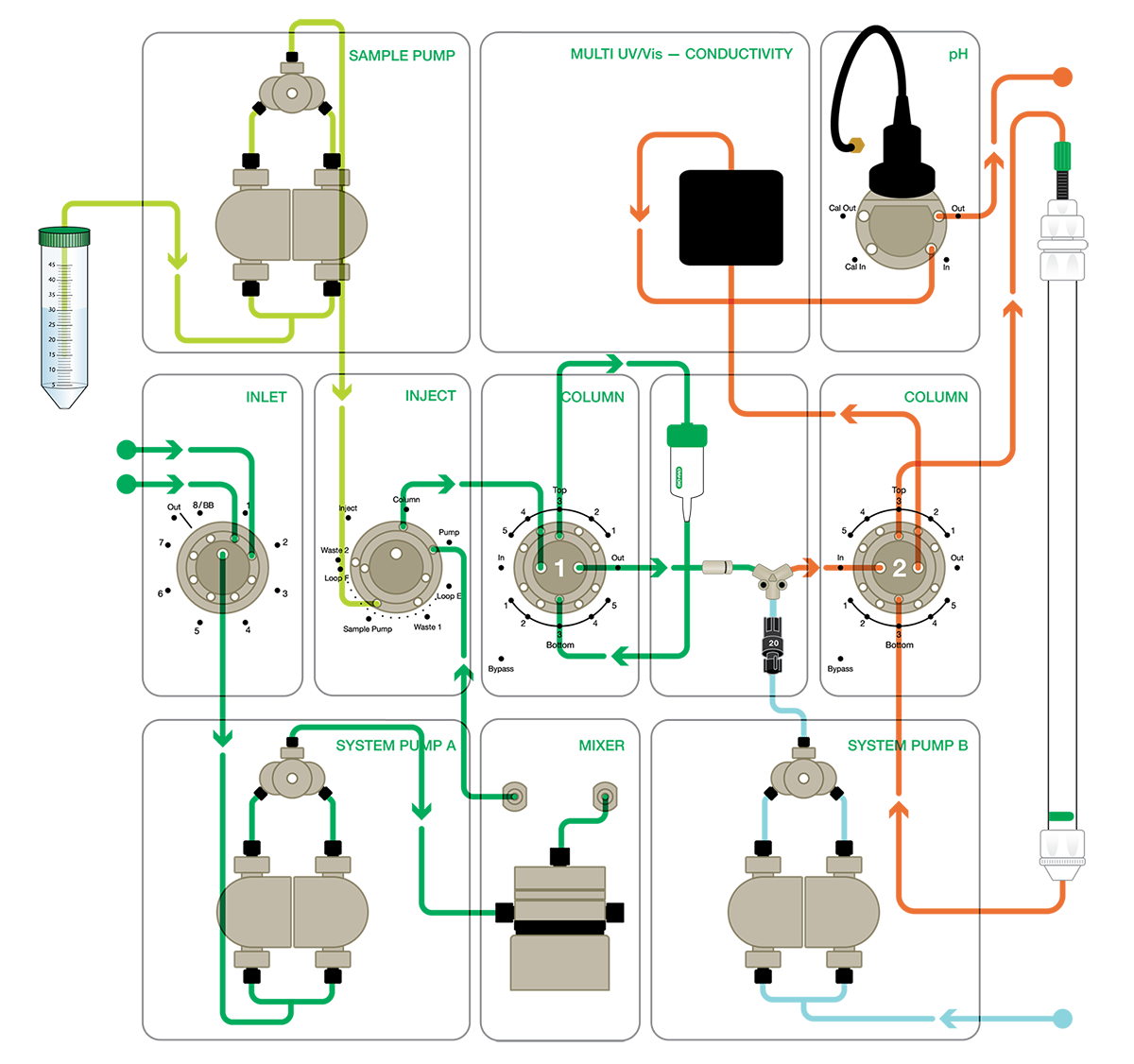
Fig. 1. NGC Chromatography System configured for tandem purification of antibodies using inline neutralization. The inlet valve is primed with two buffers: PBS, pH 7.8 (buffer 1), and 0.1 M glycine, pH 3.0 (buffer 2). System pump B delivers 1 M Tris, pH 8.0 (buffer 3), for inline pH neutralization. Arrows denote the direction of flow within each path. Sample pump flow path (■); system pump A flow path (■); system pump B flow path (■); neutralization flow path (■). PBS, phosphate buffered saline; UV, ultraviolet; Vis, visible (light).
The complete mAb purification workflow using the custom NGC Quest 100 Plus Chromatography System is illustrated in Figure 2.
To serially purify multiple antibodies automatically, an additional inlet valve placed before the sample pump serves as an autosampler and enables automated sample pump washing after each sample is introduced.
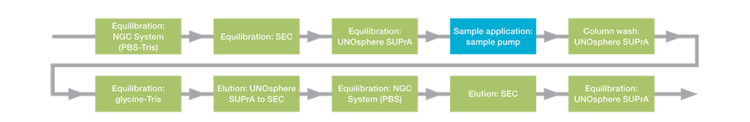
Fig. 2. ChromLab Software method outline for the tandem purification of trastuzumab.
This setup allows users to automate their antibody purification and confidently walk away, returning to high-quality antibody product. The entire two-column tandem purification workflow can be completed in less than five hours, depending on sample loading volume and whether the SEC column is pre-equilibrated. The workflow consistently and reproducibly yields pure monomeric antibodies for all of your research applications.
Want to learn more? The full workflow for automated antibody purification on the NGC System is described in this application note.
Additional Resources
Bio-Rad bulletin 3359. Inline Dilution for Antibody Purification on NGC Chromatography Systems.
Bio-Rad bulletin 3360. Configuring The NGC Chromatography System for Tandem Automated IgG Purification with Inline Neutralization: Quick Guide.
References
Hilario J et al. (2022). Automated two-column purification of trastuzumab on the NGC Chromatography System with modifications for in-line neutralization. Bio-Rad bulletin 3435.
Mazzer AR et al. (2015). Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold. J Chromatogr A 1415,83–90.




