Successful chimeric antigen receptor- (CAR-) T cell therapy requires a target antigen that is unique to cancer cells. But what happens when there are no unique antigens? Researchers at Columbia University Medical Center addressed this problem by replacing healthy non-target cells with genetically modified versions lacking the CAR-T cell target (Borot et al., 2019). Their results, published in PNAS, may provide a new avenue for treatment of some types of cancer.
CAR-T Cell Therapy and the Unique Antigen Problem
CAR-T cell therapy, an emerging cancer treatment strategy, uses T cells modified to express a CAR — a receptor engineered to recognize an antigen found on cancer cells. CAR-T cell therapy has proven highly successful for some treatment-resistant leukemias and initial studies extending the strategy to other types of cancer have been promising.
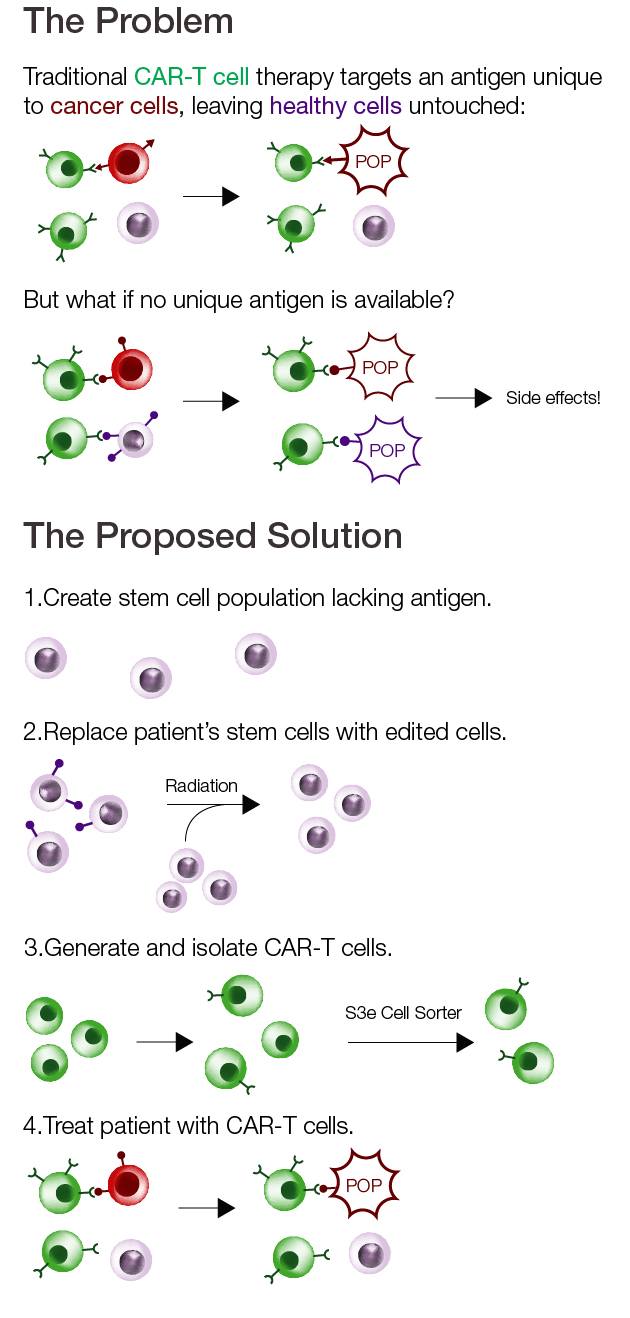
However, one of the major drawbacks for this approach is that CAR-T cells will target any cell displaying the target antigen, whether healthy or cancerous. Damage to healthy cells can result in severe side effects, such as immune cell depletion or organ damage, and even death.
Unfortunately, there are many types of cancer for which no unique antigen is known, leaving clinicians and researchers to decide whether the risk of side effects outweighs the potential benefits. This is the case for acute myeloid leukemia (AML), a type of bone marrow cancer for which new treatments are desperately needed. Immunotherapy targeting CD33, an antigen present in more than 80% of cases, has been successful for treatment of some patients with AML. However, the CD33 antigen is also found in certain immune system progenitor cells, causing severe complications in some cases.
A New Approach for CAR-T Cell Therapy
To get around the unique antigen problem, researchers Florence Borot and colleagues tested a creative solution. Rather than searching for a new antigen, they used gene editing to generate a population of healthy cells immune to the effects of CAR-T cell therapy targeting CD33.
To do this, researchers started with CD34–expressing hematopoietic stem cells isolated from bone marrow or cord blood. Using CRISPR-Cas9, expression of CD33 was ablated. Transplantation of the edited cells into mice effectively replaced the native population of bone marrow cells and their progenitors with the knockout cells, permitting safe treatment with CAR-T cells targeting CD33.
Results in vitro and in mouse models were promising, showing that the CAR-T cells successfully targeted cancer cells without affecting the edited healthy cells. These data provide hope that CAR-T cell therapy may yet be successful not only for AML, but also for other cancers faced with a lack of unique antigens.

Isolating T Cells with the S3e Cell Sorter
Researchers are increasingly incorporating a cell sorting step when generating modified cells. In this study, Borot and colleagues used a Bio-Rad S3e Cell Sorter to isolate CAR—expressing T cells after transfection. The S3e Cell Sorter is particularly well suited for experiments like these due to its ease of use and its ability to produce highly pure cell populations while preserving viability.
The S3e Cell Sorter makes cell sorting approachable for novices and experts alike. Because it has a shallow learning curve, unlike many other cell sorters, it can easily be incorporated into laboratories that do not regularly perform flow cytometry. The simple setup also reduces the risk of mistakes introduced by unnecessarily complicated equipment, saving researchers’ time and materials.
In this study, T cells isolated with the S3e Cell Sorter were injected into mice. For experiments like this one, the purity of the cells after sorting is critical. The S3e Cell Sorter produces highly pure cell populations, ensuring that contaminating cells types do not confound results or cause side effects in treated organisms. Poor purity may also lead to results that are not reproducible, wasting time and resources on downstream experiments and clinical trials.
The cell sorter must also be gentle enough to work without compromising viability. Samples that do not survive the duration of the experiment are useless regardless of purity. Stressed cells may also behave differently even if they are still viable, affecting reproducibility and confounding results. The S3e Cell Sorter was designed to handle cells gently, preserving their health and allowing researchers to experiment without worry.

