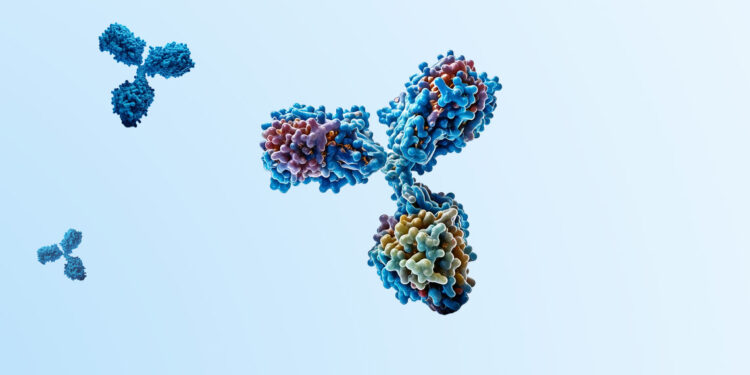
Demonstrating a Modular Selectivity with a Weak AEX-HIC Resin for Achieving High mAb Purity and Recovery in a Downstream Process

Strategies to Address Large Molecule Purification Challenges
mAb Purification Process Development Using Mixed-Mode Resins — The DOE Approach

Assessing Viral Clearance in Early-Phase Process Development
mAb Purification Process Development Using Mixed-Mode Resins — The DOE Approach
The Process of Resin Screening

Bioprocessing Recombinant Proteins

Two-Step Purification of Low-Expressing Recombinant Exoprotein A Using Mixed-Mode Chromatography

Integrating Viral Clearance into Your Process Development — A Case Study Using a Mixed‑Mode Chromatography Resin
Presented by: Akunna Iheanacho, Director of Research and Development at Texcell – North America
Xuemei He, R&D Manager, Chromatograpy Media Chemistry at Bio-Rad Laboratories
View on demand
Learn about evaluating viral clearance using a DOE approach with a mixed-mode chromatography resin.


