Protein expression analysis is essential in cell biology research, as it provides valuable insights into the molecular mechanisms that underlie cellular functions. Traditional methods for protein analysis, such as western blotting and ELISA, are useful for studying protein expression in cell lysates. However, these methods require labor-intensive sample preparation and do not provide information on the spatial distribution of proteins within intact cells. In-Cell Western is a powerful technique that enables quantitative and qualitative in situ protein expression analysis.
In-Cell Western (ICW) facilitates in situ analysis of protein expression using fixed and permeabilized cells rather than cell lysates. It enables quantification of multiple targets using spectrally distinct fluorescent dyes and is a quick method for determining relative protein levels in numerous samples. ICW is ideal for researchers who have already validated the specificity of their antibodies using traditional western blotting or for those interested in overall protein expression but don’t need to account for molecular weight.
ICW is an immunofluorescence assay combining the specificity of traditional western blotting with the higher reproducibility and output of immunofluorescence and ELISA, and can be performed in cells grown in microplates with as few as 6 or as many as 384 wells.
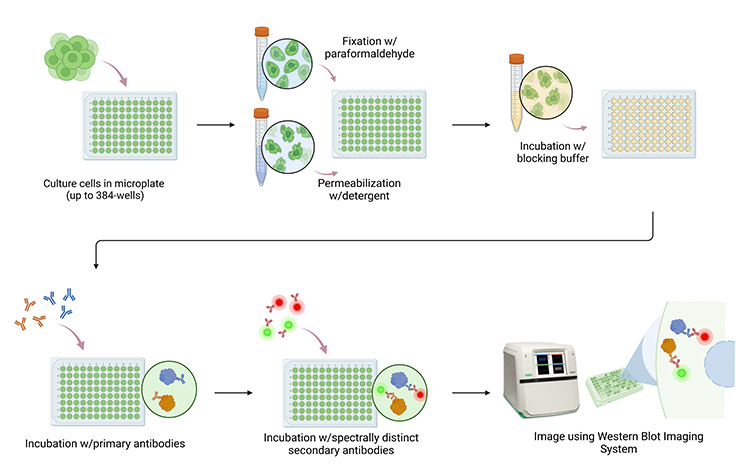
The In-Cell Western workflow. Cells are cultured in microplates with up to 384 wells, then fixed and permeabilized before being incubated with blocking buffer and antibodies. Finally, the plate is imaged using a compatible system, such as the ChemiDoc MP Imaging System.
The cells are fixed and permeabilized to allow antibody penetration when studying intracellular proteins and are probed with target-specific primary antibodies. Multiplexing capabilities are possible using spectrally distinct fluorophore–conjugated secondary antibodies. After washing away unbound antibodies, the microplate is imaged using a fluorescence-enabled western blot imager. The resulting signal intensity is proportional to the amount of target protein present in the cells. With the elimination of the sample prep, SDS-PAGE, and membrane transfer steps used in traditional western blotting protocols, ICW requires less hands-on time.
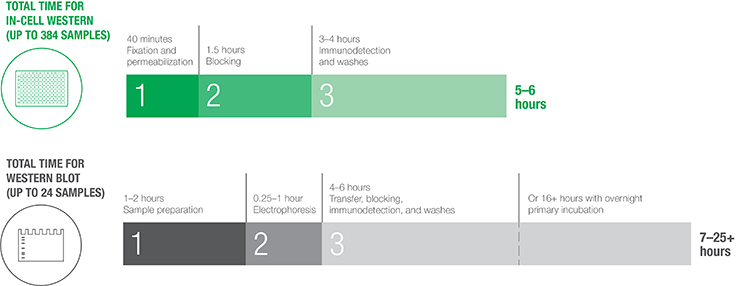
Comparison of the total time required to execute a typical In-Cell Western protocol versus a traditional western blotting protocol. While western blotting may require 7–25+ hours, with a throughput of up to 24 samples, In-Cell Western can be completed in 5–6 hours on up to 384 samples.
ICW is a versatile technique that can be used for a wide range of applications, including quantification of protein expression changes in response to drug treatments or genetic manipulations, evaluation of protein-protein interactions, and analysis of protein localization within specific cellular compartments (Moerke et al. 2011, Li et al. 2010, Aguilar et al. 2010). ICW can also be used to screen large libraries of compounds for their effects on protein expression, making it a valuable tool for drug discovery and development.
Bio-Rad’s range of products and our optimized ICW protocol can simplify and streamline your assays. Secondary antibodies conjugated to infrared or near-infrared (NIR) fluorescent dyes are commonly used for ICW multiplex detection and normalization (Pal et al., 2022). However, using Bio-Rad’s ChemiDoc MP Imaging System, researchers can leverage a broader range of fluorescently-labeled secondary antibodies that can be used in tandem with Bio-Rad’s ready-to-use hFAB Rhodamine Housekeeping Protein Fluorescent Primary Antibodies. By upgrading the base ChemiDoc System to the ChemiDoc MP System, researchers obtain a best-in-class imager that enables multiplexing of up to three targets during a single acquisition step, has five fluorescent channels, provides superior image quality, and pairs seamlessly with Image Lab Software for easy analysis.

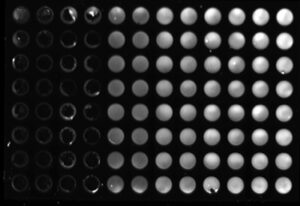


Sample images of a multiplex In-Cell Western plate imaged on the ChemiDoc MP Imaging System and analyzed using Image Lab Software. HEK293 cells were seeded in duplicate columns at 5k, 10k, 25k, 50k, 75k, and 100k cells/well and probed for Actin Gamma (MCA5776GA, DyLight 680, red), p53 (VMA00664, DyLight 800, green), and HKP control GAPDH (12004167, Rhodamine, gray).
Image Lab Software harmoniously pairs with any ChemiDoc Imager, making ICW analysis effortless with this powerful yet straightforward package. Whether using the downloadable plate templates provided on Bio-Rad’s dedicated In-Cell Western application page or creating your own using the Volume Tool, Image Lab Software automatically calculates the volume intensity of each well. When coupled with Bio-Rad’s hFAB Rhodamine Housekeeping Protein Fluorescent Primary Antibodies, you can set control and blank wells, which are used for background subtraction to calculate adjusted volume intensity.
In summary, ICW is a high-throughput tool for protein expression analysis of whole cells. With its versatility and broad range of applications, ICW is a valuable addition to any cell biology researcher’s toolkit. Bio-Rad’s portfolio of western blotting products complements the ICW workflow, making it easy to achieve high quality and accurate results.
Visit the In-Cell Western application page to learn more about how you can use the ChemiDoc MP System and Image Lab Software for your next experiment.
References
Aguilar HN et al. (2010). Quantification of rapid myosin regulatory light chain phosphorylation using high-throughput In-Cell Western assays: comparison to western immunoblots. PLoS One 5, e9965.
Li J et al. (2010). Application of glutaraldehyde to In-Cell Western assay for normalization. Anal Biochem 398, 254–256.
Moerke NJ et al. (2011). Development of In-Cell Western assays using far-red fluorophores. Curr Protoc Chem Bio 3, 39–52.
Pal A et al. (2022). In-Cell Western protocol for semi-high-throughput screening of single clones. Bio Protoc 12, e4489.


