With the advancement of technology, flow cytometers are now able to detect a much greater range of fluorescent dyes, facilitating the analysis of more extensive and complex multicolor immunophenotyping assays. But this has also introduced more pitfalls to navigate during the design of these experiments. Find out how StarBright Dyes can help circumvent many of these issues, enabling consistent and reliable results.
Immunophenotyping is a commonly used technique for studying immune cells both in basic research and clinical diagnostic labs. This method often harnesses the power of flow cytometry, using fluorescently tagged antibodies to identify cell types based on the presence or absence of defined markers. The many important applications of flow cytometry-mediated immunophenotyping include disease diagnosis, characterization of disease stage, and analysis of candidate therapeutic efficacy in translational studies.
Multicolor flow cytometry is an effective tool for delineating mixed populations of cells from blood or tissue samples, since it allows the analysis of many fluorescent markers at once. However, the advent of flow cytometers with the capacity to measure and distinguish more fluorescent dyes has been both a blessing and a curse, as it has introduced many new challenges to the creation and analysis of complex multicolor panels (McKinnon 2018).
Plan for the Brightness of Your Fluorophores
One major aspect to consider when designing your multicolor flow cytometry panel is the selection of the appropriate fluorophores for your markers. It is valuable to consider the antigen density of your target protein so you can pair difficult-to-detect markers with fluorophores with high relative brightness to maximize the resolution of rare populations. Similarly, dim fluorophores should be reserved for highly expressed markers. Fluorophore brightness/dimness can be determined using the stain index of the fluorophore, which measures the separation between the positive and negative populations (Holmberg-Thyden et al. 2021).
Consider Spectral Overlap Between Fluorophores
An additional point to consider when choosing the appropriate fluorophores for your assays is the spectral overlap of the colors used in your panel. Many fluorescent dyes can be excited by more than just their optimal laser and may have broad emission spectra, resulting in spill over into other detectors, which can dilute signal resolution. This can be corrected to a certain extent with a mathematical process known as compensation, and dyes should be chosen considering the minimum amount of compensation necessary (Roederer 2001). Ideally, the best dyes to use are those which have narrow excitation and emission spectra, and therefore are less likely to be excited by other lasers and cause spill over.
Beware of Potentially Unreliable Fluorophores
One common method that has often been used to extend the availability of novel fluorophores, is the creation of tandem dyes, where two fluorescent molecules are covalently attached to produce an emission spectrum different to that of the primary fluorophores. This broadens the range of available unique emission spectra that can be incorporated into an experiment. However, tandem dyes should be used with caution, as they can degrade and/or decouple, resulting in emission in the spectrum of the primary fluorophores. For example, in a panel where both APC and APC-Cy7 (a tandem dye) are used, degradation of the latter will result in its signal being detected as both the former and a Cy7 signal. This can significantly impact the interpretation of data, so it is best to avoid using tandem dyes when possible (Jensen et al. 2020).
Determine How Your Experimental Conditions May Impact Your Fluorophores
It is also important to consider whether it’s necessary to use any specific buffers for the fluorophores you intend to use in your panel. For example, when using polymer dyes, such as Brilliant Violet and Brilliant Ultraviolet, the use of special buffers is required to inhibit polymer-polymer interactions. Aggregation of these dyes can distort the expected fluorescent signal and cause confusion during data analysis. Not only must you pay attention to which dyes require a special buffer, but you must confirm that the other dyes in your panel are also compatible with this buffer. This becomes especially critical when building large, multicolor immunophenotyping panels due to the multitude of different types of fluorophores that will be used (Whyte et al. 2022).
Fixatives are another prime example of why you must consider the compatibility of fluorophores with your experimental conditions. Often, cells need to be fixed prior to analysis, and many fluorophores lose fluorescence when exposed to fixatives. Therefore, if you require a fixation step in your immunophenotyping protocol, it’s essential to factor this in.
StarBright Dyes: The Solution to Your Problems
While building complex, multicolor panels for immunophenotyping is challenging, the data acquired from such experiments is extensive and indispensable. Fortunately, StarBright Dyes, which have been specifically designed for multiparameter flow cytometry, address many of the common pain points associated with this technique.
Bright
StarBright Dyes are exceptionally bright, making them ideal even for low antigen density markers and rare populations. They are designed to match or exceed the brightness of their equivalent comparison dye (Figure 1).
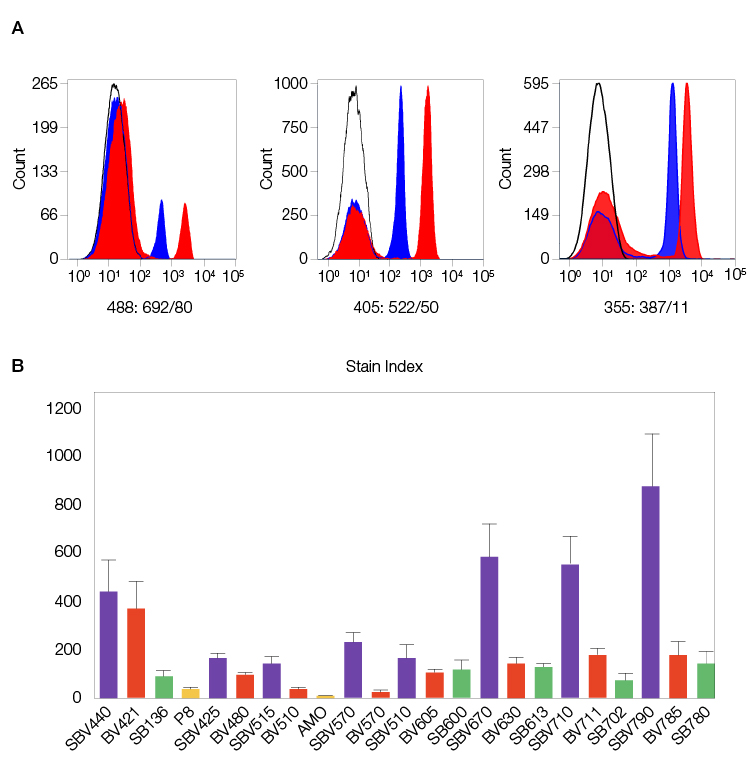
Fig. 1. StarBright Dyes are designed to match or exceed the brightness of their equivalent comparison dyes. A, examples of CD4 staining for 488, 405, and 355 nm excitable StarBright Dyes (red) compared to similar dyes (blue). B, stain index of human peripheral blood stained with various 405 nm excitable StarBright, Brilliant Violet, and Super Bright Dyes and conventional dyes conjugated to CD4. Data generated on the ZE5 Cell Analyzer. Purple, StarBright Violet Dyes; red, Brilliant Violet; green, Super Bright; yellow, non-polymer dyes. SBV, StarBright Violet Dyes; BV, Brilliant Violet; SB, SuperBright Dyes; PB, Pacific Blue.
Narrow Excitation and Emission Spectra
StarBright Dyes also have narrow excitation and emission spectra to reduce unwanted excitation by other laser lines and minimize spill over to other detectors. This simplifies compensation and makes data interpretation more straightforward when using StarBright Dyes (Figure 2).
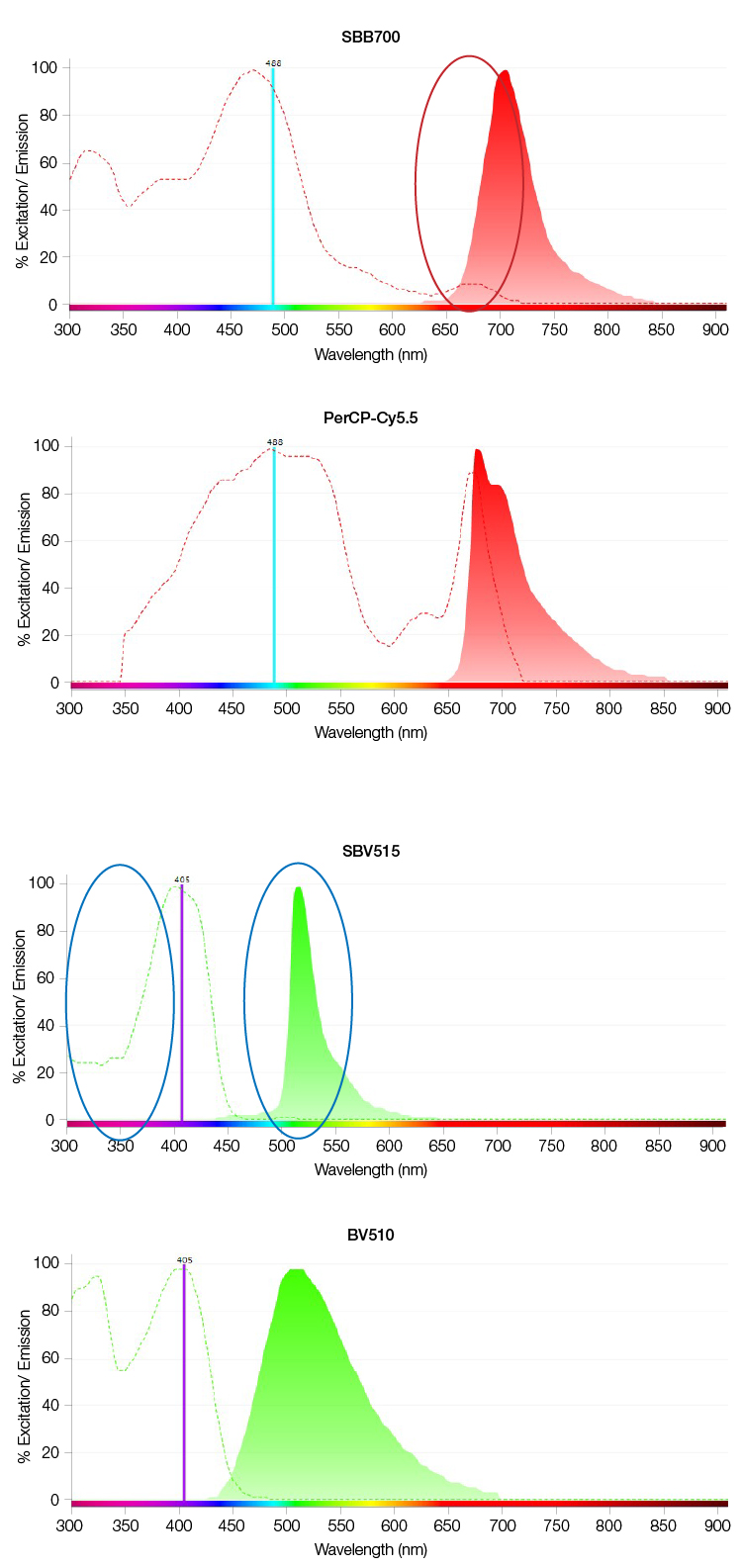
Fig. 2. Examples of improved spectral characteristics of StarBright Dyes. A, Reduced excitation of StarBright Blue 700 Dye (SBB700) compared with PerCP-Cy5.5 (red circle) by a 640nm laser, and B, reduced excitation of StarBright Violet 515 Dye (SBV515) compared to Brilliant Violet 510 (BV510) (blue circles) by a 355 nm laser with narrow emission for SBV515.
Compatible with All Buffers
Another key feature of StarBright Dyes is their flexibility. Not only do they not require a special buffer, they are also unimpacted by the presence of special buffers required by other dyes in your panel, producing bright and reliable staining (Figure 3).
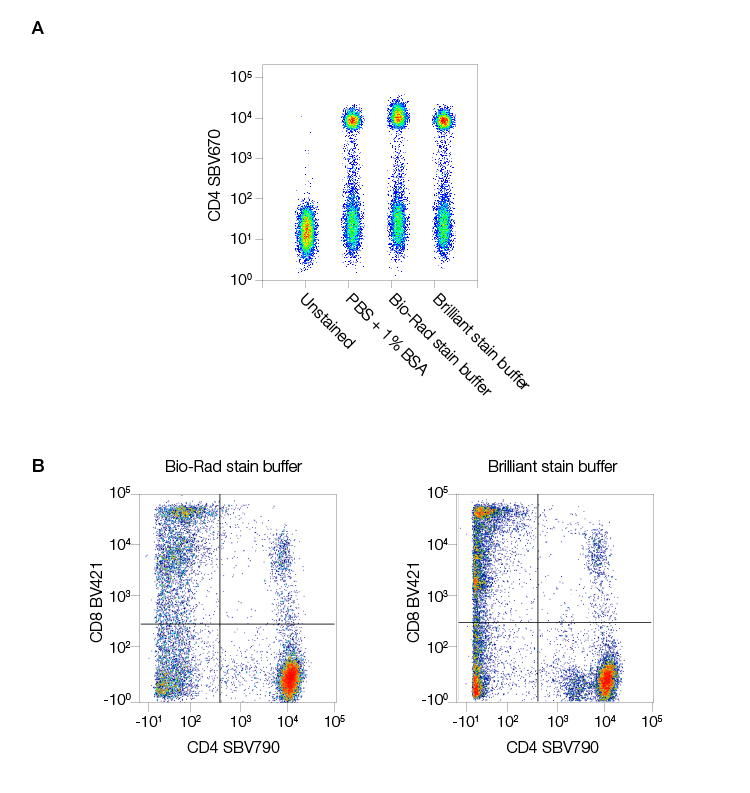
Fig. 3. StarBright Dyes are compatible with all buffers. Staining human peripheral blood in different buffers revealed A, no change in CD4 staining with StarBright Violet 670 Dye (SBV670) (MCA1267SBV670) regardless of the buffer used and that B, StarBright Dyes (in this case StarBright Violet 790 Dye [SBV790] [MCA1267SBV790]), work well both alone in Bio-Rad Staining Buffer or with one Brilliant Violet Dye in Brilliant Staining Buffer. Data generated on the ZE5 Cell Analyzer.
Compatible with All Instruments and Reagents
StarBright Dyes are compatible for immunophenotyping on any flow cytometer with suitable lasers and filters and are suitable for use alongside all fluorescently tagged antibodies.
Consistent Staining
StarBright Dyes are not traditional tandem dyes, so they are less likely to experience cross-laser excitation. Their stability means they give consistent staining and results over time. Even fixation with paraformaldehyde (PFA)-based or alcohol-based fixatives will not alter the brightness of their signal (Figure 4).
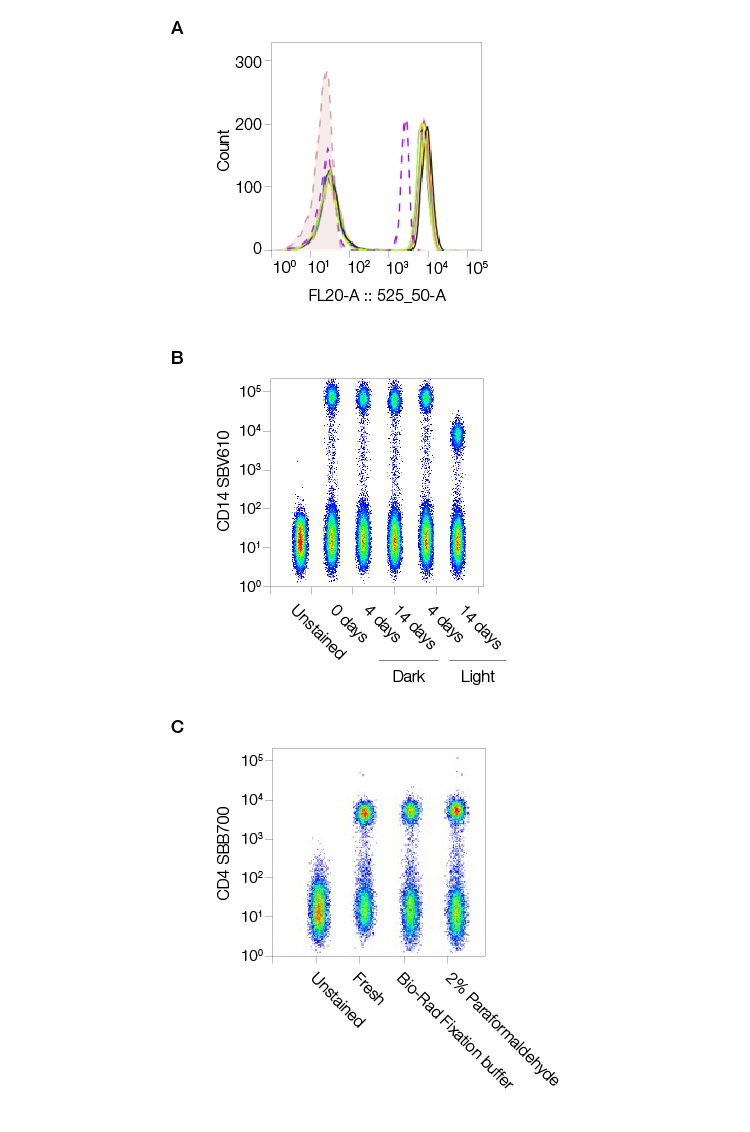
Fig. 4. StarBright Dyes are consistent, stable, and unaffected by fixation. StarBright Dyes yield consistent results regardless of lot. A, multiple StarBright Dye (CD4SBV515) (MCA1267SBV515) lots were used to stain peripheral blood mononuclear cells with, and the results overlaid on the histogram (dotted line) produced by staining with CD4SBV510 for comparison. B, StarBright Dye (CD14SBV610) (MCA16568SBV610) was left at room temperature in the dark or in the light for up to 14 days prior to staining human peripheral blood, and the resultant stain was compared to that achieved with antibody stored correctly in the fridge. A reduction in staining was only seen after 14 days storage at room temperature in the light. C, Fixation in Bio-Rad Fixation Buffer or 2% PFA in phosphate-buffered saline (PBS) has no effect on the stain index of CD4SBV515 compared to freshly stained samples. Data generated on the ZE5 Cell Analyzer.
Conjugated with Common Target Antibodies
StarBright Dyes conjugated to highly validated antibodies for common immunophenotyping targets, specifically well-known, highly cited clones, are also available, giving you greater flexibility in your panel design (Figure 5).
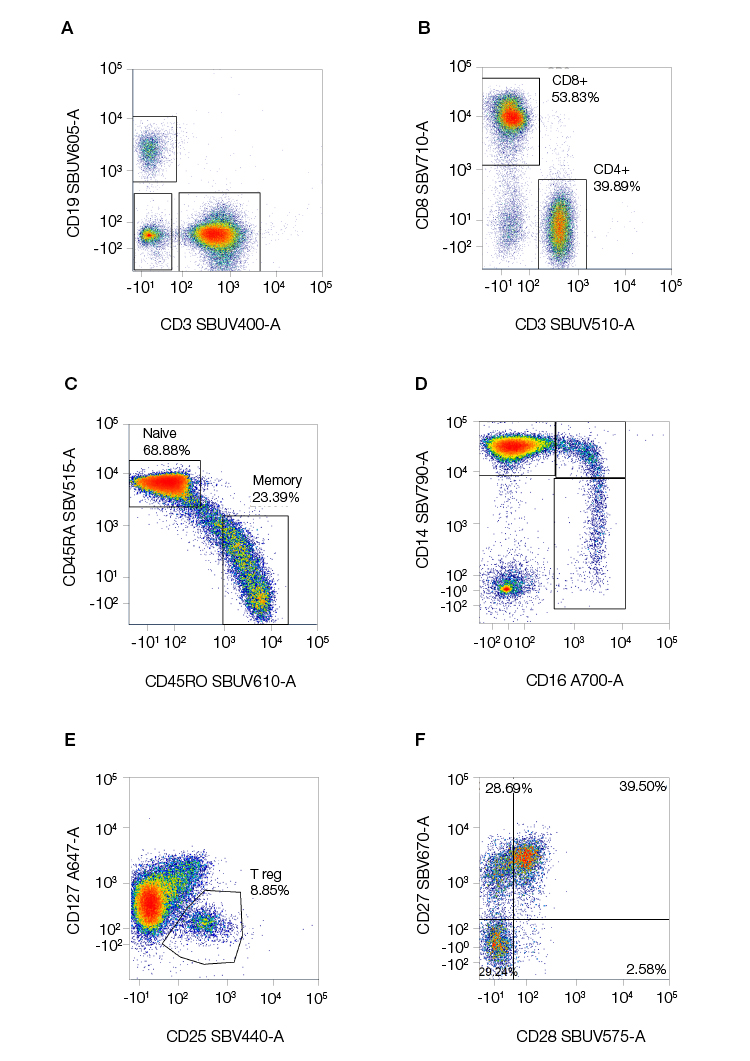
Fig. 5. Examples of staining of common immunophenotyping markers using StarBright Dyes. Human peripheral blood was stained for A, CD3 (MCA463SBUV400), CD19 (MCA1940SBUV605), B, CD4 (MCA1267SBUV510), CD8 (MCA1226SBV710), C, CD25 (MCA2127SBV440), and CD127 (HCA145A647; discontinued) to identify B and T lymphocytes, and T helper, T cytotoxic cells, and T regulatory cells within the T cell population. D, Classical, intermediate, and nonclassical monocytes were identified using CD14 (MCA1568SBV790 and CD16 (MCA2537A700). E, Further characterization of the T helper cells was obtained from CD45RA (MCA88SBV515) and CD45RO (MCA461SBV610) to identify naïve and memory cells F, the co-stimulation marker expression on T regulatory cells was measured by CD27 (MCA755SBV670) and CD28 (MCA709SBUV575) levels. Data generated on the ZE5 Cell Analyzer.
StarBright Dyes, with their unmatched brightness, narrow excitation and emission spectra, compatibility with buffers and fixatives, and availability for a wide range of common immunophenotyping targets are an excellent solution for counteracting the challenges associated with building multicolor flow cytometry panels for immunophenotyping. With StarBright Dyes, researchers can reproducibly obtain consistent and reliable immunophenotyping data.
References
Holmberg-Thyden S. et al. (2021). A user’s guide to multicolor flow cytometry panels for comprehensive immune profiling. Anal Biochem 627, 114210.
Jensen I. et al. (2020). Worry and FRET: ROS production leads to fluorochrome tandem degradation and impairs interpretation of flow cytometric results. Immunity 52, 419–421.
McKinnon K. (2018). Multiparameter conventional flow cytometry. Methods Mol Biol 1678, 139–150.
Roederer M. (2001). Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry 45, 194–205.
Whyte C. et al. (2022). Do more with less: Improving high parameter cytometry through overnight staining. Curr Protoc 2, e589.

