The ZE5 Cell Analyzer offers the speed and enhanced reproducibility to enable new approaches as well as improvements on current protocols. The technology facilitates deeper characterization of cell populations and superior rare event analysis timelines. Offering significant expansion of capabilities over traditional flow cytometers, the ZE5 Cell Analyzer can advance flow cytometry for numerous applications.
Attain Unmatched Speed and Stability
The ZE5 Cell Analyzer combines improved speed, enhanced electronics, and a cell laser transit time that is up to five times faster (Figure 1) than other systems’ to create a unique and indispensable cell analysis system for any lab. It can run at high speeds without compromising data quality due to enhanced fluidics that deliver a stable flow rate of up to 3.5 μl/sec. To match this rapid flow rate, the ZE5 Cell Analyzer’s faster laser transit time creates a shorter pulse width, which is sampled two times more frequently than comparable systems. The increased sampling rate, coupled with fast, low-noise electronics, results in a tighter coefficient of variation (CV) and higher-resolution data (Figure 2).
The speed of the ZE5 Cell Analyzer enables more in-depth investigation and more specific analysis in applications such as identification of specific cell subsets undergoing apoptosis, real-time activation and proliferation of cells due to immune or drug response, or detection of cells in different stages of the cell cycle.
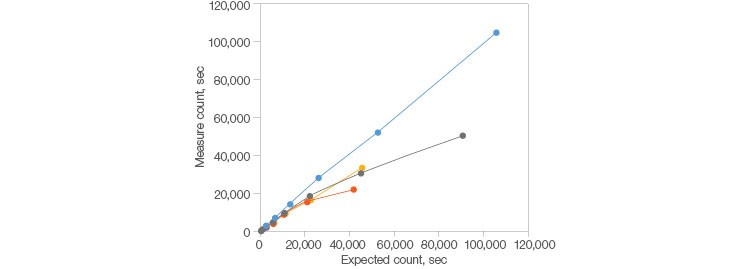
Fig. 1. Demonstrated longevity. The ZE5 Cell Analyzer outperforms other systems at higher acquisition speeds as it continues to acquire data into the 100,000 events per second range whereas other systems drop off around 20,000 events per second. Dragon Green Beads (Bangs Laboratories, Inc.) were used in a serial dilution to determine when the observed acquisition rate falls off the theoretical limit. ZE5 Cell Analyzer (●); cytometer 1 (●); cytometer 2 (●); cytometer 3 (●).
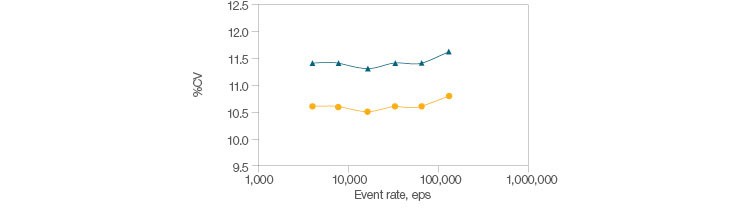
Fig. 2. Signal stability with high acquisition. Beads were read at increasing events-per-second rates, and singlet beads were gated using pulse geometry gating. The %CV of side scatter and FITC were plotted over approximately 4,000–129,000 eps. The mean and standard deviation of CVs over this range are shown below. FITC-A (●), μ = 10.6, s = 0.063; SSC-A (▲), μ = 11.4, s = 0.063.
Analyze Rare Cell Populations
The ZE5 Cell Analyzer makes rare cell sorting possible with more detectors, greater speed, and minimal electronic noise levels.
More detectors
With up to five lasers and 30 detectors, the ZE5 Cell Analyzer easily improves upon standard assays, supports new panels, and is an excellent aid in difficult-to-study rare cell analysis. The option for multiple spatially separated lasers facilitates the study of highly complex samples. Numerous detectors expand options and allow for larger panels, enabling critical data acquisition of whole samples. Samples no longer need to be split, thereby increasing the sensitivity of measurement.
Speed and stability
The blend of speed and stability found in the ZE5 Cell Analyzer is well suited for rare event analysis. In order to measure a cell found at a frequency of 1 in 105 cells, 40 million events need to be analyzed to collect 400 positive events. The analysis of 40 million events with a typical flow cytometer running 10,000 events per second is estimated at 150 minutes (2.5 hours) for a single sample. The ZE5 Cell Analyzer can acquire the same amount of data in less than 10 minutes. At 100,000 events per second, the ZE5 enables rapid and thorough analysis and characterization of even the rarest cell populations (Figure 3).
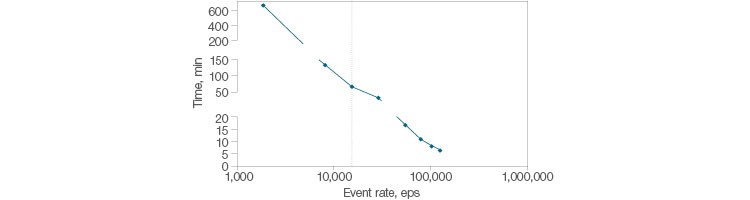
Fig. 3. Fast acquisition for rare cells. Time to collect 400 positive events of a rare population (1 in 105 cells) is plotted versus the speed of acquisition (events per second). Collecting 40 million events with a typical flow cytometer takes 150 minutes (2.5 hours) for a single sample. The ZE5 Cell Analyzer can acquire data at up to 100,000 events per second.
Minimal electronic noise
Low electronic noise allows identification of dim populations faster by increasing sensitivity of detection and incorporating higher resolution. The ZE5 Analyzer has less electronic noise on the low end than the BD FACSymphony Analyzer, which makes it easier to identify these dim populations (Figure 4).
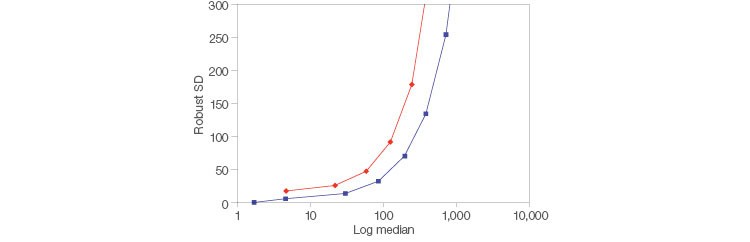
Fig. 4. Reduced noise for easier detection. Two-peak beads (Spherotech) were run on a ZE5 Cell Analyzer and BD FACSymphony Cell Analyzer (BD Biosciences). FlowJo Software was used to determine the median and robust SD for the peak at each voltage and for FITC. ZE5 Cell Analyzer (■); BD FACSymphony Analyzer (◆).
Study Small Samples with Ease
The ZE5 Cell Analyzer has small particle detection capability (FSC from the 405 nm laser), permitting analysis of difficult-to-study samples such as exosomes, which are generally smaller than 250 nm (Figure 5). Exosomes are extracellular vesicles (EVs) that have been implicated in intracellular communication and are a key area of interest in disease research. Conventional exosome analysis using flow cytometry can require manual hardware adjustments and advanced instrument calibration. The ZE5 Cell Analyzer now provides a user-friendly platform with built-in capabilities that allow any lab to study these and other small particles. Using both surface and internal markers, centrifugation, beads, or other staining methods, the ZE5 displays optimal sensitivity for small particle identification.
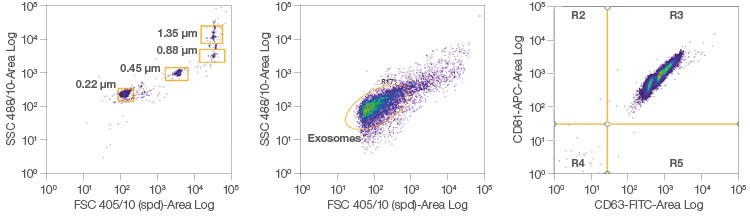
Fig. 5. Exosome analysis. Exosomes from MCF-7 cells were stained for CD63 and CD81. Staining for more than one target allows for the identification of subpopulations of exosomes using the ZE5 Cell Analyzer.
Obtain Better Reproducibility with No Carryover
Conventional flow cytometry requires manual washing of the probe between samples to reduce sample to sample carryover. The ZE5 Cell Analyzer automates this activity with its flying collar wash feature that automatically washes the probe between samples. Automation of crucial steps reduces potential contamination, thus removing a primary source of data variation (Figure 6), and provides results you can trust.
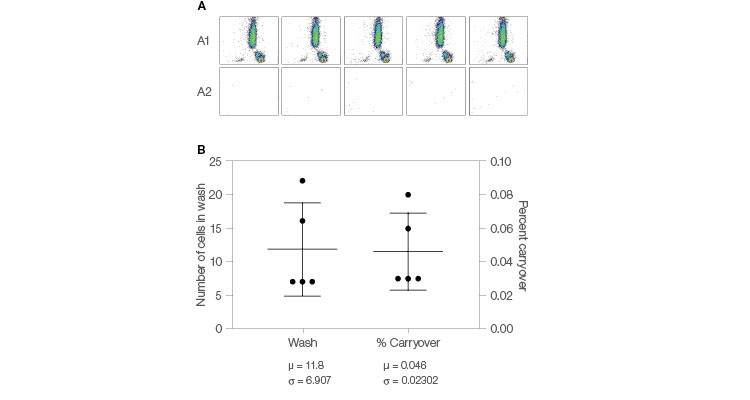
Fig. 6. Carryover reduction. A, lysed whole blood was run on the ZE5 Cell Analyzer in high-throughput (HT) mode. After each sample, the system performed an automatic wash cycle of 0.25 sec outside and 1.75 sec inside the sample injection port (SIP). A clean tube of water was run immediately after the wash to evaluate carryover. B, the resulting carryover data show an average carryover of 0.046% (± 0.023%). Carryover = (CountWash Well / CountSample Well) x 100
Get Great Resolution and Reproducibility
The low-noise electronics on the ZE5 Cell Analyzer also contribute to great resolution and consistent, reproducible data even at the high speeds only the ZE5 Cell Analyzer can achieve (Figure 7).
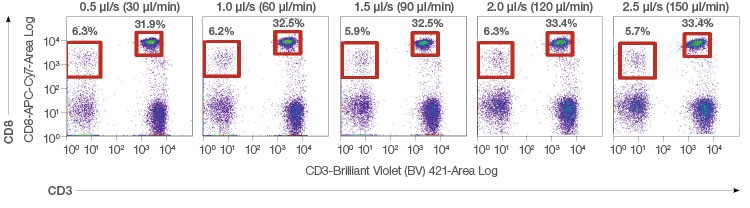
Fig. 7. Robust reproducibility. Whole blood was lysed and stained with CD45-Alexa Fluor 488/CD3-BV421/CD8-APC-Cy7. 20,000 events were acquired per run to evaluate various population percentages over multiple event rates.
Publish, Don’t Re-Run Experiments
The ZE5 instills confidence in results and enables quicker experiment to publication time. With reduced carryover, faster speed of acquisition, and multiple parameters, the ZE5 Cell Analyzer supports the robust exploration of novel assays while improving the quality and reproducibility of ongoing experiments.
FACSymphony is a trademark of Becton Dickinson and Company. FlowJo is a trademark of FlowJo, LLC. Alexa Fluor is a trademark of Thermo Fisher Scientific Inc. Cy is a trademark of GE Healthcare.

