Abstract
High purity protein is a common requirement for biochemical and structural studies. A common approach is to recombinantly express an affinity-tagged version of the protein of interest. This is, however, not always a viable option. In this report we discuss protein purification workflow development for untagged proteins and introduce a new indicator of method performance, the purity quotient difference (PQD). We show a case study on the process of optimization of the purification workflow for the untagged prancer purple protein by scouting for the optimal resin, pH, and %B using the NGC chromatography system.Introduction • Materials and Methods • Results • Discussion
Figures
Protein Purification Workflow Optimization
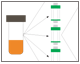 Fig.1. Scouting steps during protein purification.
%B Scouting
Fig.1. Scouting steps during protein purification.
%B Scouting
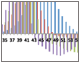 Fig. 4. Identification of optimal elution buffer concentration
Fig. 4. Identification of optimal elution buffer concentration
Column Scouting
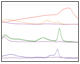 Fig. 2. Optimization of resin, SDS-PAGE analysis, and evaluation.
Final Purification Scheme for Untagged Prancer Purple Protein
Fig. 2. Optimization of resin, SDS-PAGE analysis, and evaluation.
Final Purification Scheme for Untagged Prancer Purple Protein
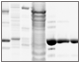 Fig. 5. SDS-PAGE analysis of the purified untagged prancer purple protein.
Fig. 5. SDS-PAGE analysis of the purified untagged prancer purple protein.
pH Scouting
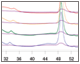 Fig. 3. Optimization of pH and analysis.
Fig. 3. Optimization of pH and analysis.
Introduction
High purity protein samples are essential for applications from protein structure determination and biochemical characterization to antibody production. A standard purification approach is the use of affinity tags such as hexahistidine (6x histidine) or glutathione-S-transferase (GST) tags. These tags increase the throughput and efficiency of the protein purification workflow, as protocols are often readily available or supplied by the manufacturer. Affinity tagging is not always a viable option. Some proteins are unstable or inactive once tagged or require posttranslational modifications that do not permit recombinant expression. In these cases, researchers often settle for lower purity protein rather than exhaustively explore purification options, since the purification optimization process can be time and labor intensive when no particular column resins or buffer conditions are dictated by an affinity tag. Whether purifying tagged or untagged proteins, an optimal purification workflow includes column, pH, and elution buffer gradient (%B) optimization for each purification step (Figure 1). Here we show that the Bio-Rad NGC medium-pressure chromatography system, equipped with column switching valve, sample pump, buffer blending valve, and the ChromLab™ software scouting feature, allows us to automate the process of column, pH, and %B optimization. Using the untagged, chromogenic protein, prancer purple, we illustrate that small changes in column chemistry or pH have drastic effects on protein binding and changes in the elution buffer pH or gradient can greatly impact purity of the eluted protein. Our findings cement the importance of column, pH, and buffer scouting when developing purification workflows and illustrate how the Bio-Rad NGC chromatography system and ChromLab software facilitate this process and make untagged proteins of high purity attainable for every researcher.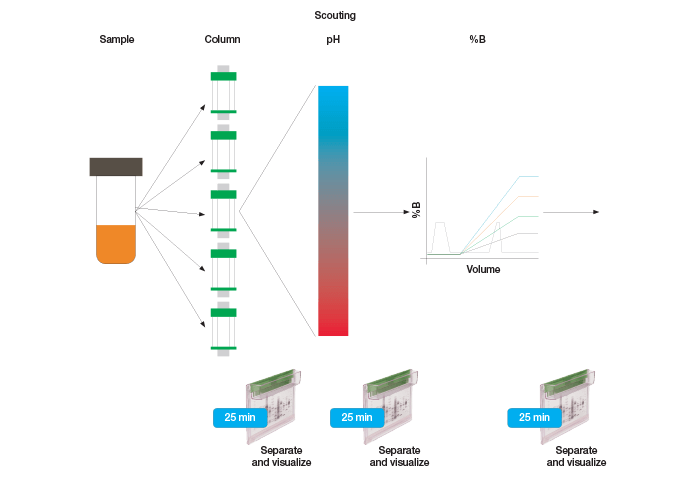
Fig. 1. Protein purification workflow scouting.
Materials and Methods
Protein Expression The kanamycin-resistant prancer purple expression vector (DNA 2.0) was transformed into HB101 E.coli using the standard heat shock method. A single colony was picked into 10 ml LB supplemented with kanamycin (LB/kan) and grown overnight at 37°C. Cells were then pelleted using an Eppendorf 5804 centrifuge (rotor A-4-44) at 4,000 rpm (2,880 RCF) for 10 min, resuspended in 10 ml of fresh LB/kan, and inoculated into 1 L of LB/kan. Cultures were grown at 37°C on a shaker set to 200 rpm. Prancer purple expression was induced when cultures reached an OD600 of 0.4, by adding IPTG to a final concentration of 0.1 mM. After overnight induction, cells were harvested by centrifugation with a Sorvall SA-600 rotor at 10,000 rpm (14,500 RCF) for 10 min. Kanamycin was used at a concentration of 50 μg/ml for liquid and solid phase growth. Cell Lysis Cell pellets were resuspended in 20 ml B-Per (Pierce Cat #78243) lysis buffer supplemented with 20 μl of 1 mg/ml DNase I and 200 μl of 1 M MgCl2. Lysates were clarified by centrifugation at 10,000 rpm (14,500 RCF) for 10 min. Column Scouting Bio-Rad’s NGC Discover™ system was used for all chromatography. Three 1 ml anion exchange chromatography columns, Foresight™ Nuvia™ Q, Bio-Scale™ Mini UNOsphere™ Q, and Bio-Scale™ Mini Macro-Prep® High Q, and a 5 ml Bio-Scale™ Mini CHT™ Type II chromatography column were attached to different positions on the NGC column switching valve. The ion exchange scouting method was generated in ChromLab software. Using the buffer blending valve, stock solutions Q1 (0.2 M HCl), Q2 (0.2 M Tris), Q3 (water), and Q4 (4 M NaCl) were mixed to generate buffer A (50 mM Tris pH 7.5) and buffer B (50 mM Tris pH 7.5 and 1 M NaCl). The scout feature was used to scout four columns attached to different positions of the column switching valve. Clarified lysate was diluted threefold with water and 1 ml of diluted lysate was directly injected onto the columns using the sample pump. Columns were equilibrated for 5 column volumes (CV) with buffer A. Approximately 1 ml of sample was applied to the columns. The columns were then washed with 5 ml of buffer A and protein was eluted with a 20 CV linear gradient from 0–50% B followed by a 10 CV 50% B wash. After the linear gradient the columns were stripped and re-equilibrated with a 5 CV 100% B wash, followed by a 10 CV buffer A wash. All purification steps were carried out at a 1 ml/min flow rate. Fractions from each run were analyzed by SDS-PAGE. pH Scouting Once the Foresight Nuvia Q column was selected as the first column, the ChromLab software scout feature was set to the pH scouting option. Binding was assessed at pH 7.0, 7.5, 8.0, and 8.5. 1 ml of diluted lysate was directly injected onto the Foresight Nuvia Q column at each pH during the method using the sample pump. Approximately 0.2 ml fractions were collected from each run and analyzed by SDS-PAGE. %B Scouting After pH 7.5 was selected, the scout feature was used to optimize %B. The 10 CV linear gradient portion of the elution phase was selected for the %B scouting (20, 30, 40, and 50 final %B). 1 ml of diluted lysate was directly injected onto the Foresight Nuvia Q column at each pH using the sample pump. 0.2 ml fractions were collected from each run and analyzed by SDS-PAGE. CHT Ceramic Hydroxyapatite Chromatography The pooled Foresight Nuvia Q fractions were diluted twofold with water. The buffer system on the Bio-Rad NGC Discover system was changed to phosphate (Q1 = 0.2 M sodium phosphate monobasic and Q2 = 0.2 M sodium phosphate dibasic) and 10 ml of sample were loaded onto a 5 ml Bio-Scale Mini CHT Type II column pre-equilibrated with 50 mM sodium phosphate pH 7. Protein was eluted with a 20 CV linear gradient from 0–50% B and 1 ml fractions were collected. ENrich™ SEC 70 Chromatography The pooled CHT fractions were concentrated to 200 μl (~20-fold) using a Millipore 5k concentrator. The sample was then injected via static loop onto an ENrich SEC 70 size exclusion column pre-equilibrated with 50 mM sodium phosphate and 150 mM NaCl (15% B). Protein was eluted using isocratic flow for 1.25 CV. 1 ml fractions were collected and analyzed by SDS-PAGE. SDS-PAGE Analysis For all chromatography scouting, samples were analyzed by SDS-PAGE on Any kD™ Criterion™ TGX Stain-Free™ polyacrylamide gels. Gels were run for 30 min at 300 mA/gel and then immediately imaged using the ChemiDoc™ imaging system. Purity Quotient Calculations Data for individual scouting runs were opened in the evaluation tab of the ChromLab software (Figure 2C). Peak integration was performed on the 280 and 525 nm traces. The table in the fractions tab containing peak area and peak relative area was imported into a Microsoft Excel spreadsheet. The purity quotient (PQ) for each wavelength was calculated by dividing the relative area (%) by the collected volume (ml). The purity quotient difference for prancer purple (PQDPP) was calculated for each fraction as PQ525 – PQ280 and graphed as a histogram. Histograms of the runs for each scouting type (column, pH, and %B) were graphed together for analysis. PQD equations: PQ = Relative Area (%)/Collected Volume (ml) PQPOI – PQcontaminant = PQD PQD > 0: more of the protein of interest (POI) than contaminants PQD < 0: more contaminants than POI PQD = 0: POI is present in equal amount as contaminants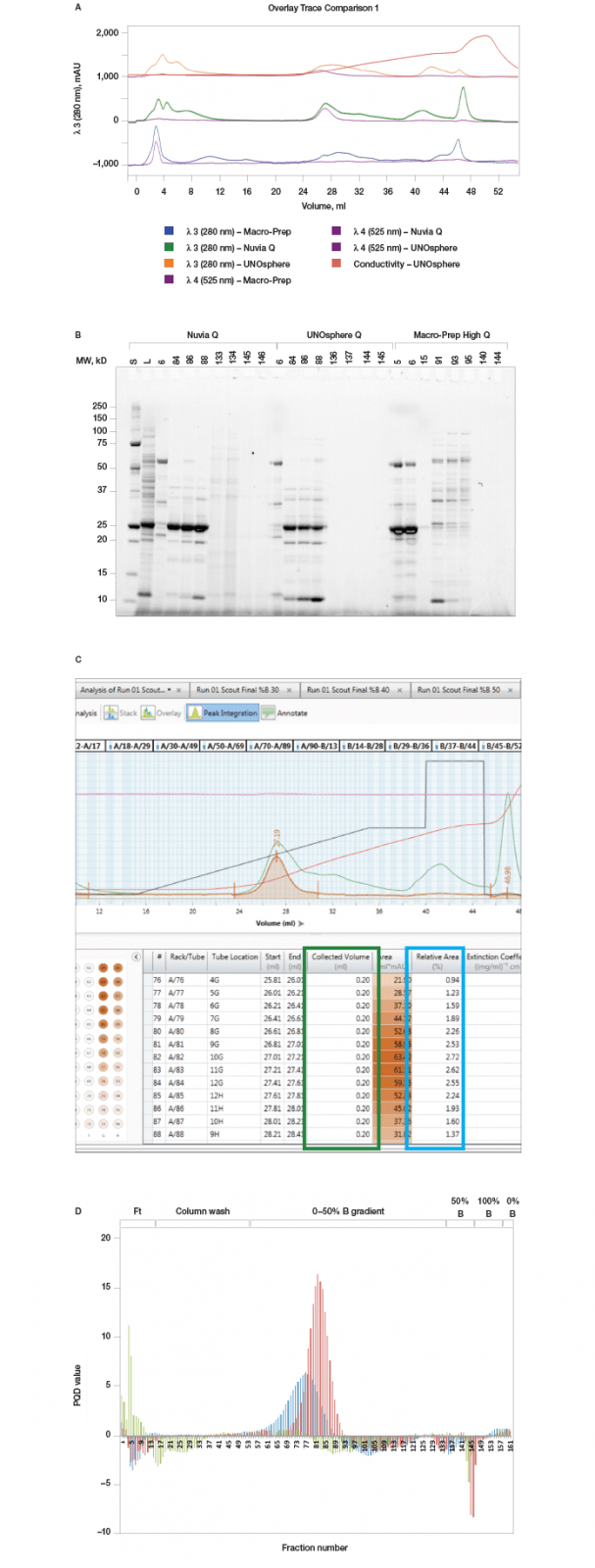
Fig. 2. Column scouting. A, offset chromatogram overlays of prancer purple column scouting runs. Total protein, 280 nm traces of Macro-Prep Q (■), Nuvia Q (■), and UNOsphere Q (■), runs are overlaid with the prancer purple absorbance trace at 525 nm (■). B, SDS-PAGE analysis of column scouting. 20 μl of each fraction was loaded on a 26-well Any kD Criterion TGX Stain-Free SDS-PAGE gel. The prancer purple E.coli lysate (L) was diluted 1:10 before loading 20 μl. Prancer purple molecular weight: 26.4 kD. S, Precision Plus Protein™ Unstained standard. C, ChromLab software evaluation of the Nuvia Q column scouting run. The fractions tab in the peak integration menu shows the 525 nm fraction heat map and data table. The table contains the calculated relative area and collected volume for each fraction, which is used to calculate the purity quotient. D, histogram of the prancer purple purity quotient difference (PQ525 – PQ280) per fraction from each column scouting run. UNOsphere Q (■); Nuvia Q (■); Macro-Prep Q (■).
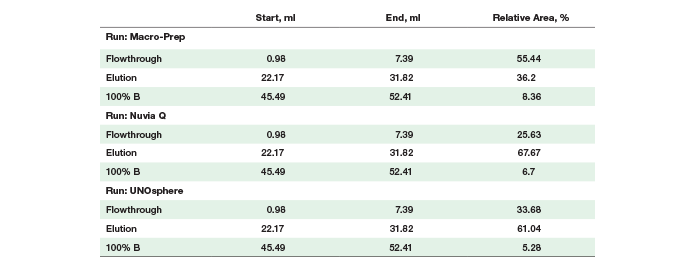
Prancer Purple Binding % Calculations Individual column scouting runs were opened in the evaluation tab of the ChromLab software. Peak integration using default settings was performed on the 525 nm trace. Switching to the manual integration tab, the peak list for each run was reduced to three peaks (flowthrough, elution, and 100% B) with the following start and end volumes: flowthrough 0.98–7.39 ml, elution 22.17–31.82 ml, and strip 45.49–52.41 ml.Results
Column Scouting Identifies Optimal Resin for Protein Binding Untagged prancer purple was expressed recombinantly in E.coli. Prancer purple has a predicted isoelectric point (pI) of 6.65 and the chosen lysis buffer a pH of 7.5, making anion exchange an excellent first step in our purification workflow. Bio-Rad has a number of different anion exchange resins of various bead sizes and chemistries. We chose a larger bead size that could accommodate faster flow rates with our highly viscous crude lysate sample. Three 1 ml anion exchange chromatography columns, Foresight Nuvia Q, Bio-Scale Mini UNOsphere Q, and Bio-Scale Mini Macro-Prep High Q, were attached to different ports of the NGC column switching valve. A single method was generated in ChromLab software specifying identical buffer conditions and flow rate for all three columns. Selecting the column variable in the ChromLab software scout feature allowed us to assess protein binding under identical sample and chromatography conditions. An automated queue of the three runs was made possible by using the NGC sample pump to directly inject the sample onto the column during the sample application phase and by automatically assigning collected fractions during elution to the appropriate column scouting run. Even though all three resins are anion exchange resins, marked differences in their ability to bind prancer purple were observed, most likely due to differences in bead chemistries. Looking at the relative area percentages (Table 1), prancer purple bound most efficiently to Foresight Nuvia Q resin, less efficiently to Bio-Scale Mini UNOsphere Q resin, and did not bind to Bio-Scale Mini Macro-Prep High Q resin (Figure 2). The calculated purity quotient difference for prancer purple (PQDPP) and SDS-PAGE data supported the conclusion that Foresight Nuvia Q was the best resin choice for the first step of the prancer purple purification workflow.Table 1. Prancer purple relative area percentages
 pH Scouting Identifies Optimal Binding pH
With the best column resin chosen, we next optimized the method’s buffer pH. Since pH affects the ionization status of proteins, it is a particularly crucial factor in ion exchange chromatography. Utilizing the buffer blending valve and the pH scouting option in the ChromLab software scout feature, we tested binding of prancer purple to Foresight Nuvia Q resin over a pH range from 7.0–8.5 in an automated queue of four runs. Although prancer purple bound to the Foresight Nuvia Q resin at every tested pH, at higher pH more contaminants bound to the columns (Figure 3). To minimize contaminants, a buffer pH of 7.5 was chosen.
pH Scouting Identifies Optimal Binding pH
With the best column resin chosen, we next optimized the method’s buffer pH. Since pH affects the ionization status of proteins, it is a particularly crucial factor in ion exchange chromatography. Utilizing the buffer blending valve and the pH scouting option in the ChromLab software scout feature, we tested binding of prancer purple to Foresight Nuvia Q resin over a pH range from 7.0–8.5 in an automated queue of four runs. Although prancer purple bound to the Foresight Nuvia Q resin at every tested pH, at higher pH more contaminants bound to the columns (Figure 3). To minimize contaminants, a buffer pH of 7.5 was chosen.
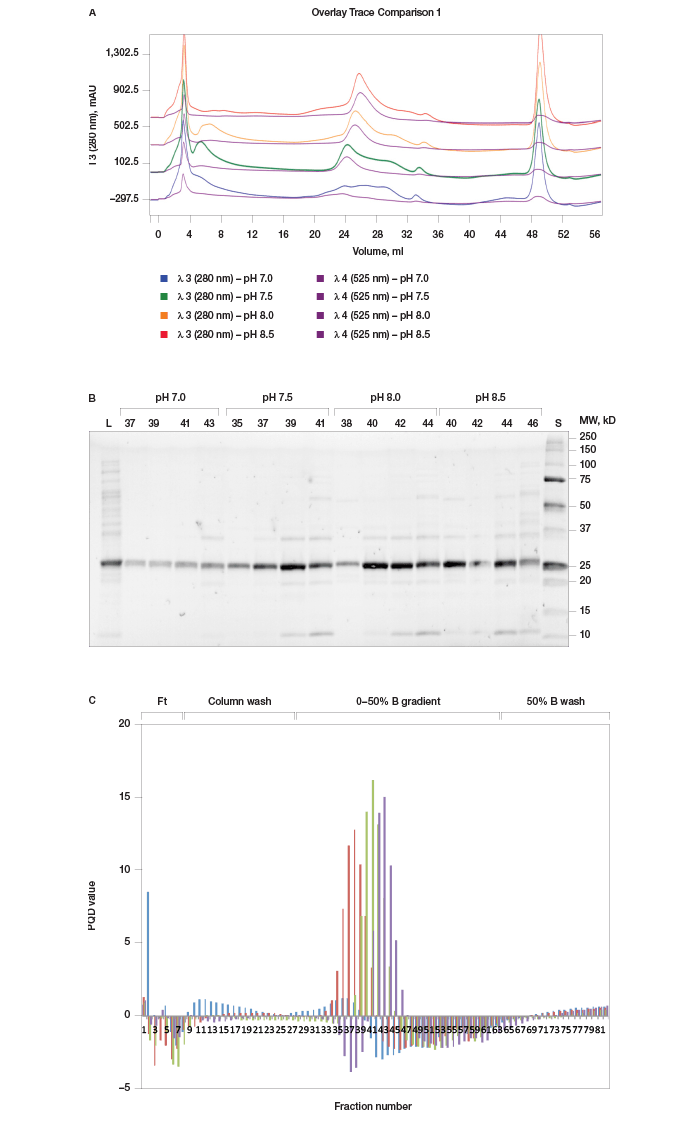
Fig. 3. pH scouting. A, offset chromatogram overlays of prancer purple pH scouting runs. Total protein, 280 nm traces of pH 7.0 (■), 7.5 (■), 8.0 (■), and 8.5 (■) are overlaid with the prancer purple absorbance trace at 525 nm (■). B, SDS-PAGE analysis of pH scouting. 20 μl of each fraction was loaded on an 18-well Any kD Criterion TGX Stain-Free SDS-PAGE gel. The prancer purple E.coli lysate (L) was diluted 1:10 before loading 20 μl. Prancer purple molecular weight: 26.4 kD. S, Precision Plus Protein Unstained standard. C, histogram of the prancer purple purity quotient difference (PQ525 – PQ280) per fraction from each pH scouting run. pH 7.0 (■), 7.5 (■), 8.0 (■), and 8.5 (■).
%B Scouting Identifies Optimal Elution Buffer Concentration and Gradient Once an optimal pH was established for prancer purple binding, we optimized the elution step to separate prancer purple from remaining contaminants. Using the %B scouting option of ChromLab software, we assayed a 10 CV elution gradient from 20–50% final %B in four separate runs. The shallowest buffer gradient, 0–20% B, resulted in optimal enrichment of prancer purple (Figure 4A, B). Some high and low molecular weight contaminants were still present, leading us to add additional purification steps to our workflow (Figure 4B).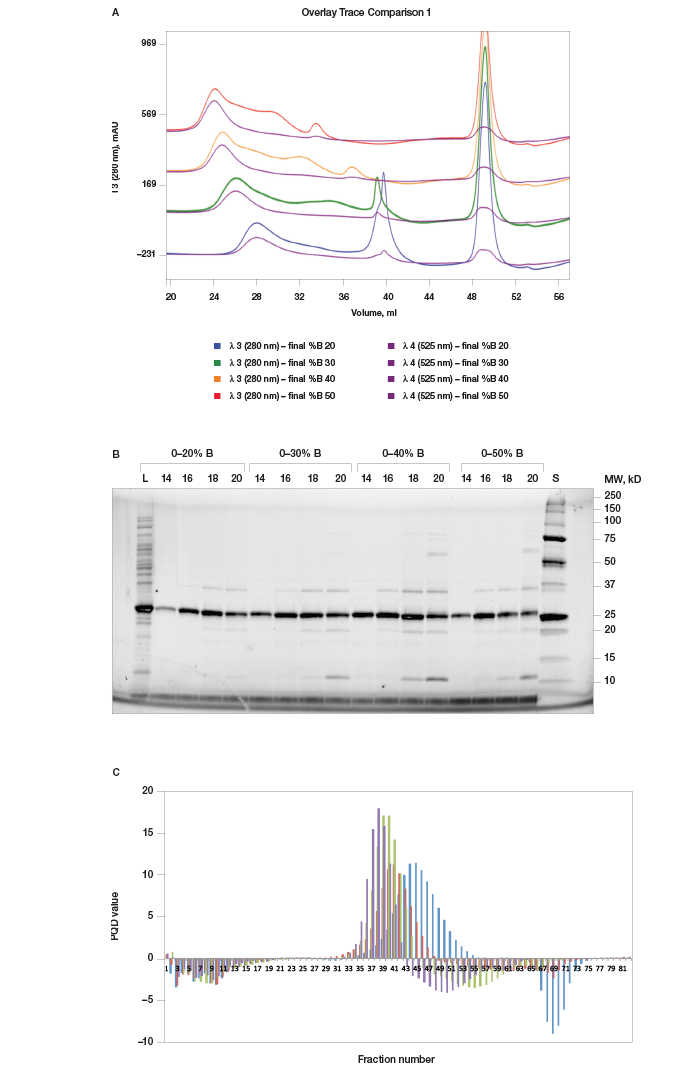
Fig. 4. %B scouting. A, offset chromatogram overlays of prancer purple %B scouting runs. Total protein, 280 nm traces of the 20 CV gradient elution to a final %B of 20% (■), 30% (■), 40% (■), and 50% (■) are overlaid with the prancer purple absorbance trace at 525 nm (■). B, SDS-PAGE analysis of %B scouting. 20 μl of each fraction was loaded on an 18-well Any kD Criterion TGX Stain-Free SDS-PAGE gel. The prancer purple E.coli lysate (L) was diluted 1:10 before loading 20 μl. Prancer purple molecular weight: 26.4 kD. S, Precision Plus Protein Unstained standard. C, histogram of the prancer purple purity quotient difference (PQ525 – PQ280) per fraction from each %B scouting run. 20% B (■), 30% B (■), 40% B (■), and 50% B (■).
Elimination of Remaining Contaminants Using Bio-Scale Mini CHT Type II Ceramic Hydroxyapatite Resin During our initial column scouting phase, we also assessed prancer purple binding to the mixed-mode ion exchange resin Bio-Scale Mini CHT Type II ceramic hydroxyapatite. Prancer purple showed high affinity for this resin and markedly, the contaminants in the elution profile were distinct from those found in our Foresight Nuvia Q elution. We therefore chose Bio-Scale Mini CHT Type II resin for our second purification step, to remove remaining contaminants from the Foresight Nuvia Q elution. The optimized Foresight Nuvia Q–Bio-Scale Mini CHT Type II column combination yielded highly pure prancer purple as illustrated by the absence of contaminating bands in Any kD Criterion TGX Stain-Free polyacrylamide gels (Figure 5). Buffer Exchange and Sample Polishing Using Size Exclusion Chromatography For some applications, such as crystallography, final elution buffer composition is crucial. Similarly, many applications require the final sample buffer composition to be identical from batch to batch. When using gradient elution, however, the exact buffer composition of the pooled elution fractions is frequently unknown. Size exclusion chromatography (SEC) is an excellent method to ensure consistent buffer composition from prep to prep, since fractionation is not dependent on buffer gradients. SEC also eliminates low abundance contaminants, proteolyzed fragments, and aggregate species, ensuring high purity and uniformity of final protein. We therefore incorporated a final size exclusion step in our purification protocol using the ENrich SEC 70 column, which has a high pressure limit that allowed higher flow rates and reduced the time needed for this step compared to carbohydrate based beads (Figure 5).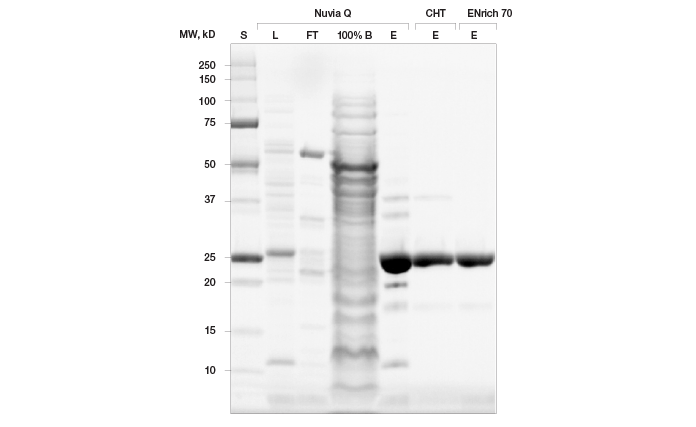
Fig. 5. Final purification workflow for untagged prancer purple protein. 20 μl of sample was loaded on to an Any kD Criterion TGX Stain-Free SDS-PAGE gel. Lysate (L) and Nuvia Q flowthrough were diluted 1:20. Elutions (E) are shown for Nuvia Q, CHT Type II ceramic hydroxyapatite, and ENrich 70 columns. Prancer purple molecular weight: 26.4 kD.
Discussion
This study illustrates that untagged protein can be purified to high homogeneity when purification options are sufficiently explored. Exhaustive exploration is often impractical, because identifying an ideal purification workflow requires optimization of multiple variables, such as column resin, pH, and %B, at each step. This means that a multitude of runs is required at each purification step to identify the purification workflow that yields protein of the desired purity. Traditionally, this process requires individually set up and programmed runs for each tested column, pH, and %B condition. ChromLab software, however, allowed us to automate and accelerate this tedious process. ChromLab software can be programmed to execute several runs sequentially. It controls different components of the NGC system, such as the NGC sample pump that directly injects sample onto the column, the buffer blending valve that allows scouting of pH and %B, and the column switching valve that can automatically switch between up to five different columns. This permitted us to scout four ion exchange columns without user intervention in the same setup and instrument programming time as that required for one traditional run. In a similar fashion, we were able to explore multiple pH conditions without the need to make and titrate individual buffers for each tested pH. Finally, automated %B scouting allowed us to improve the final purity of protein. Our data show that column scouting is essential, even when a specific resin type, such as anion exchange resin, has already been chosen. As we show here, resins designed for similar use (in this case anion exchange) can show drastically different binding efficiencies for a given protein. Even though Foresight Nuvia Q and Bio-Scale Mini UNOsphere Q resins are both strong anion exchange resins with trimethylamine functional groups, prancer purple bound only to Foresight Nuvia Q resin. This is most likely due to differences in bead chemistries and size. A second challenge of purification workflow development is evaluating which of the scouted methods yields the best results. Traditionally, based on the A280 trace of the chromatogram, which tracks the presence of protein eluting from the column throughout the chromatography run, fractions are chosen for analysis by SDS-PAGE. A method is then chosen that (1) yields high amounts of the protein of interest, (2) yields few contaminating proteins, and (3) elutes the protein of interest over a small volume. Here we introduced a third factor, the purity quotient difference (PQD), that can inform method development when the protein of interest and contaminants absorb light at different wavelengths. Most proteins contain tryptophans and thus absorb light at a wavelength of 280 nm. Chromogenic proteins, fluorescently tagged proteins, or metal-bound proteins such as hemoproteins, as well as contaminants such as DNA, absorb light at other wavelengths. Using the NGC Discover multi-wavelength detection system, we could distinguish the elution profile of prancer purple (525 nm) from the elution profile of contaminating proteins (280 nm). Because the area under the absorbance peaks in a chromatogram is proportional to the amount of protein eluted in that peak, we were able to calculate the purity quotient difference for prancer purple. The peak integration feature of ChromLab software calculates the area under elution peaks, as well as the relative peak area, which is a measure of what percentage of total protein of interest loaded elutes in a particular peak (Figure 2C). Since we wanted to determine which method yielded the highest concentration of prancer purple, we normalized the relative peak area by dividing it by the volume collected. We call this value the purity quotient and it was calculated for prancer purple (PQ525) and total protein (PQ280). The goal of preparative chromatography, however, is not simply to obtain a high yield of protein; the goal is to obtain a high yield of pure protein. Purity is traditionally assessed qualitatively by SDS-PAGE. By subtracting the purity quotient of the contaminant (PQ280) from the purity quotient of the protein of interest (PQ525), we calculated the purity quotient difference for prancer purple (PQDPP). Using these calculations, a PQD of zero indicates that contaminants and the protein of interest are present at equal amounts. A PQD >0 indicates that the protein of interest is enriched, and a PQD PQD analysis, however, is intended to complement, not replace, SDS-PAGE. Although a high PQD indicates enrichment of the protein of interest, it does not preclude the presence of contaminating proteins. The PQD histograms for pH scouting would suggest that there is no improvement in purification when the pH is raised to 8.0 from 7.5 (Figure 3C). SDS-PAGE analysis, however, clearly shows that more contaminants co-elute with prancer purple at pH 8.0 (Figure 3B). PQD histograms also do not address the nature of contaminants. Our initial round of column scouting included the mixed-mode Bio-Scale Mini CHT Type II ceramic hydroxyapatite resin. We found that prancer purple was purified to a similar extent using the Foresight Nuvia Q and Bio-Scale Mini CHT Type II resins but different contaminating proteins were found in the elutions. We were thus able to identify an optimal second column for prancer purple purification in our initial column scouting runs. This emphasizes the need to analyze not only the final purity of the protein of interest but also the impurities present in each column scouting run, as this information can inform the choice of resin for subsequent purification steps. Using Bio-Rad’s precast Any kD Criterion TGX Stain-Free polyacrylamide gels we were able to resolve and analyze protein samples in less than 30 minutes, which permitted us to characterize elution patterns from a large number of fractions in a very short time. The combination of SDS-PAGE and PQD analysis described here can be applied to column, pH, and %B scouting, as well as additional scouting steps, such as optimization of organic phase for reverse-phase chromatography or ammonium sulfate concentration for hydrophobic interaction chromatography. As this prancer purple case study illustrates, NGC system-mediated automation of these scouting processes allowed exhaustive exploration of purification options. Combined with ChromLab software’s intuitive programming features and preloaded purification templates, the NGC system allowed us to purify an untagged protein without compromising purity.Legal Notices
B-PER is a trademark of Thermo Fisher Scientific.
Microsoft and Excel are trademarks of Microsoft Corporation.
Precision Plus Protein standards are sold under license from Life Technologies Corporation, Carlsbad, CA, for use only by the buyer of the product. The buyer is not authorized to sell or resell this product or its components.

