Just as good things come in small packages, small vesicles promise big biological impact
The Nobel Prize Committee certainly recognized the value of small vesicles when it awarded the 2013 Nobel Prize for Medicine to Professors Randy Schekman, James Rothman, and Thomas C. Südof for their respective roles in discovering the regulatory mechanisms of small vesicles in cell-to-cell communication (Balch et al. 1984, Kaiser and Schekman 1990, Perin et al. 1990). These Nobel Prize–winning studies paved the way for further insight into how vesicle transport relates to disease processes. We now know that defective vesicle transport is linked to a number of diseases such as diabetes, as well as neurological and immunological disorders (Gissen and Maher 2007).
Small vesicles are nano-sized membrane sacs released by cells that can transfer biological information to other cells (Edgar 2016). They can be considered a snapshot of the cell’s contents as they often contain cellular proteins, RNA, and mRNA. These extracellular entities come in three primary flavors — exosomes, microvesicles, and apoptotic bodies — and range in size from 30 to 2,000 nm in diameter (Willms et al. 2016). Once considered cellular waste, these vesicles are now appreciated as therapeutic treasure with the potential to revolutionize the treatment, monitoring, and diagnosis of diseases such as cancer.
Yet, despite the fact that extracellular vesicles were first recognized over 50 years ago, several questions still remain with regard to fully understanding their biological significance (Edgar 2016). This may be due partly to their small size compared to cells, which makes studying them a major challenge.
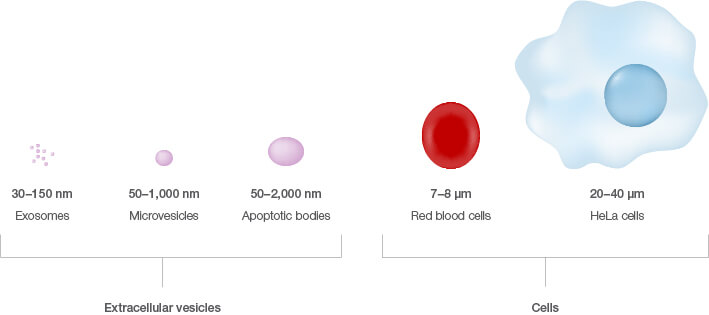
Size comparison of extracellular vesicles versus cells.
The many ways to study small vesicles
Dr. Ankur Kulshreshtha of the CSIR-Institute of Genomics and Integrative Biology (IGIB; Delhi, India) studies exosomes with the goal of specifically targeting cells and tissues for various therapeutic purposes. To characterize exosomes, he has used serial centrifugation, density gradient centrifugation, dynamic light scattering (DLS), transmission electron microscopy (TEM), and nanoparticle tracking analysis (NTA). These are common techniques for determining the size, concentration, and morphology of small vesicles. However, they are not ideal for complex analyses. According to Dr. Kulshreshtha, “the biggest challenge with all of them remains multiplexing. They become impractical when your sample numbers are [greater than] 100.”
Multiplexing allows the quantitative measurement of multiple parameters, which can provide significant insight into the biology of small vesicles. This is a major limitation of common assays such as electron microscopy. While western blotting and real-time quantitative PCR allow for simultaneous analysis of the expression of multiple proteins and RNA in extracellular vesicles, respectively, these assays require extensive effort to first isolate the small vesicles (Pedersen et al. 2015).
Ultracentrifugation, density gradients or cushions, size exclusion, and precipitation are the most common methods for isolating small vesicles, particularly exosomes. However, they are all labor intensive and present increased risk of both loss of purified sample and contamination by protein/lipid aggregates (Pedersen et al. 2015). Furthermore, they do not allow for high-throughput analysis and extensive characterization of small vesicles.
Therefore, of the many ways to study small vesicles, flow cytometry has emerged as a valuable technique for gaining further insight beyond size and morphology, as well as for fast and simple isolation.
For small vesicles, it’s best to just go with the flow
Using flow cytometry to study exosomes, Dr. Kulshreshtha has observed that “sample processing is easy, and you can go for higher throughputs. Also, you don’t need any exotic reagents.” Beyond these benefits, when it comes to studying extracellular vesicles, flow cytometry also allows for quantitative protein characterization with minimal bias (Wu et al. 2016).
In contrast to ultracentrifugation, flow cytometry can be used to distinguish small vesicle subtypes based on specific protein markers (Willms et al. 2016). It is therefore possible to isolate exosomes from cell culture supernatant containing apoptotic bodies and microvesicles using antibodies to common exosome markers, such as CD9, CD63, CD81, ALIX, or TSG101 (Edgar 2016, Pedersen et al. 2015, Willms et al. 2016). Flow cytometry–based isolation also reduces the loss of purity observed with ultracentrifugation (Pedersen et al. 2015).
Magnetic capture beads are often used to facilitate analysis of exosomes in flow cytometry as the size of the beads compensates for limits of detection that otherwise let small vesicles avoid detection. But it is possible to abandon capture beads and detect small vesicles directly. The ZE5 Cell Analyzer from Bio-Rad eliminates the need for capture beads thanks to its built-in small particle detector, which allows analysis of samples in the nanometer range. Using size beads, it can quickly be set up to readily collect small vesicles.
The ZE5 Cell Analyzer addresses another limiting factor of flow cytometry in the analysis of small vesicles, this one to do with the sheath. The sheath commonly used in standard flow cytometers often contains debris and small particles that can be mistaken for extracellular vesicles, as their sizes are similar. To avoid inaccurate detection, sheath purification would need to be performed prior to analysis. The ZE5 Cell Analyzer utilizes DI water as its sheath and has an inline sheath filter that eliminates the need to prefilter. It must be noted that samples should also be filtered prior to running on any flow cytometer to help ensure purity of the sample and confidence in the analysis of small vesicles.
Learn more about exosome analysis on the ZE5 Cell Analyzer and hear how a Bio-Rad scientist sets up the ZE5 Cell Analyzer to detect exosomes directly.
Taken together, the main advantages of using flow cytometry to study small vesicles include increased processing speed, capacity for high-throughput quantitative analysis with minimal bias, and the ability to use off-the-shelf antibodies.
Other advantages that Dr. Kulshreshtha enjoys are that “it saves time and effort and allows one to be more productive.”
“We have just started exploring these enigmatic vesicles. They have the potential for transforming early diagnosis of diseases as well as for targeted delivery of biologics,” says Dr. Kulshreshtha on the future of research on small vesicles. When it comes to making the great discoveries that allow small vesicles to fulfill their promise of huge biological and therapeutic impact, flow cytometry will certainly be a key player.
Download a PDF of this article.
References
Balch WE et al. (1984). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39, 405–416.
Edgar JR (2016). Q&A: What are exosomes exactly? BMC Biol 14, 46.
Gissen P and Maher ER (2007). Cargos and genes: Insights into vesicular transport from inherited human disease. J Med Genet 44, 545–555.
Kaiser CA and Schekman R (1990). Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61, 723–733.
Pedersen KW et al. (2015). Direct isolation of exosomes from cell culture: Simplifying methods for exosome enrichment and analysis. Transl Biomed 6, 1–9.
Perin MS et al. (1990). Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263.
Willms E et al. (2016). Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6, 22519.
Wu Y et al. (2015). Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 140, 6,631–6,642.
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions. All trademarks used herein are the property of their respective owner.

