When a photo-excitable molecule absorbs light energy and subsequently emits light energy, this is known as fluorescence. More specifically, some molecules can be excited to a higher energy state by absorbing light (excitation). However, this excited state cannot be sustained for a long time so the energy decays and the molecule returns to its ground state by releasing light energy. This emission of light energy is known as fluorescence (Figure 1).
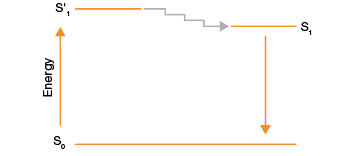
Fig. 1. Jablonski diagram of the fluorescence process. Molecule S in its ground state (S0) absorbs light energy and attains an excited state, S’1. The energy at this state decays quickly and brings the molecule to a less excited state (S1). Molecule S eventually returns to its ground state, emitting the excess energy as light or fluorescence.
Need an easy way to pick the right fluorophore for your next fluorescent western blot?
Get our handy fluorophore reference poster for western blotting and gel imaging.

Fluorescent dyes or fluorophores are molecules that have the ability to absorb light energy at a particular wavelength and emit light at a lower energy and thus longer wavelength. This typically means that the color of light absorbed is different from the color of light emitted. Theoretically, fluorophores are able to undergo the cycle of absorbing light, becoming excited, and emitting light indefinitely. Since this means that a fluorophore molecule can produce a continuous fluorescence signal that can be collected in a cumulative way by a detector, fluorescence is considered to be a very sensitive technique. In essence, fluorescence intensity remains linear over a relatively long period of time and over many orders of magnitude of the expression level of a protein of interest.
Fluorescent dyes are able to absorb light over a range of wavelengths and each has a characteristic excitation range, called its excitation spectrum. However, certain wavelengths within the excitation range cause maximum excitation of the molecule; this peak is called the excitation maximum (Ex). Likewise, fluorescent dyes also have an emission spectrum that covers the range of wavelengths that the molecule emits light at. The emission maximum (Em) is the wavelength at which the molecule fluoresces most intensely. The difference in wavelength between the excitation and emission maxima reflects the Stokes shift and is a characteristic of the fluorophore (Figure 2).
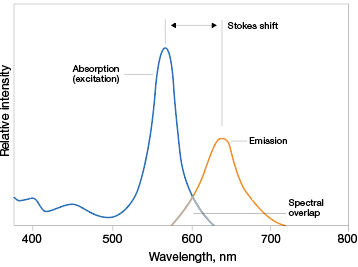
Fig. 2. Properties of fluorophores. Fluorophores absorb and emit light over a range of wavelengths. The wavelengths at which they most effectively absorb and emit light are known as their excitation and emission maxima, respectively. The difference between these two wavelengths is known as Stokes shift.
Fluorescence-based techniques have become increasingly popular, and among them is fluorescent western blotting. Learn more about fluorescent western blotting and how it can enhance your western blotting process. However, before you can start your fluorescent western blotting experiments, it is vital that you understand how to pick the fluorophores for your specific needs.
Picking the Right Fluorophore
1
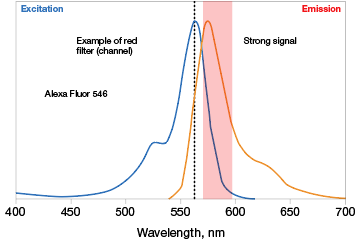
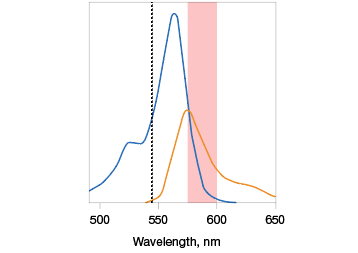
Know the excitation and emission maxima of the fluorophores you wish to work with. Some fluorescent molecules have sharp and narrow Ex/Em scans while some have wider, flatter scans. The optics (excitation light source and emission filters) of your imaging instrument have to work with these properties, so it is critical to know these before picking fluorophores (Figure 3).
A. Optimal excitation
532 laser
A. Sub-optimal excitation
514 laser
B.
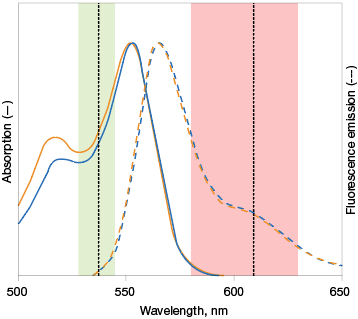
Fig. 3. Optimal fluorophore excitation and emission. A, optimal excitation (top) occurs when the fluorophore picked has an excitation maxima that is compatible with the excitation light source of the imaging instrument. Otherwise, a fluorophore may be excited in a suboptimal manner (bottom), decreasing the intensity of the emission maxima measured. B, selecting fluorophores with overlapping excitation spectra can lead to difficulty in teasing out the emission signal of one from the other. It is important to pick fluorophores that do not excite with the exact same excitation source to the same extent. Alexa Fluor (—); Cy3 (—).
2
Pick fluorophores with a wide Stoke’s shift since, for a particular fluorescent molecule, the wider the Stoke’s shift, the less the spectral overlap, and the cleaner the data (Figure 2).
3
When using two or more fluorophores in the same experiment, make sure they are mutually compatible. The more spectrally separated the Ex/Em of each fluorophore are from the other, the better and easier quantitation will be. For multiplexing, it is best to pair fluorophores that have Ex/Em spectra with minimal overlap (that is, in the area under the curve).
Get information about Bio-Rad’s new StarBright™ and hFAB™ Fluorescent Antibodies and ChemiDoc™ MP Imaging System, designed for multiplex detection, brighter signal, and a streamlined workflow.
Alexa Fluor is a trademark of Life Technologies Corporation. Cy is a trademark of GE Healthcare.

