Co-immunoprecipitation (co-IP) is a good tool for identifying target protein complexes and interacting partners. Though highly informative, co-IP is also a challenging technique to master.
The following six tips will help you achieve successful co-IP results with SureBeads Magnetic Beads.
1. Samples
- Select biologically relevant samples that have your target protein complex
- Avoid high concentrations, harsh detergents like SDS, and additives that will cause protein complex dissociation
2. Immunoprecipitation
- Maintain protein complexes by using freshly prepared lysates
- Select capture reagent based on the host species of the capture antibody. Protein A has higher affinity for rabbit IgGs, while Protein G has higher affinity for mouse IgGs
3. Unidirectional Co-IP
- Due to differences in abundance, you may observe co-IP in one direction, rather than two. When this occurs, immunoprecipitate the component that has the highest percentage of its total population bound to its partner
4. Other Antibodies
- Try another antibody if you do not observe co-IP. Only unbound protein will immunoprecipitate if the epitope of the capture antibody overlaps the protein-protein interaction site
5. Positive and Negative Controls
- To show that the IP worked, include a blot for just the immunoprecipitated component (positive control)
- To show that the co-IP worked, include a blot for just the SureBeads Magnetic Beads or IgGs (negative control)
6. Analysis
- To avoid antibody detection in the western blot, use a detection antibody from a different host species than the antibody used in the IP
The following results were achieved using these tips and following the protocol as outlined:
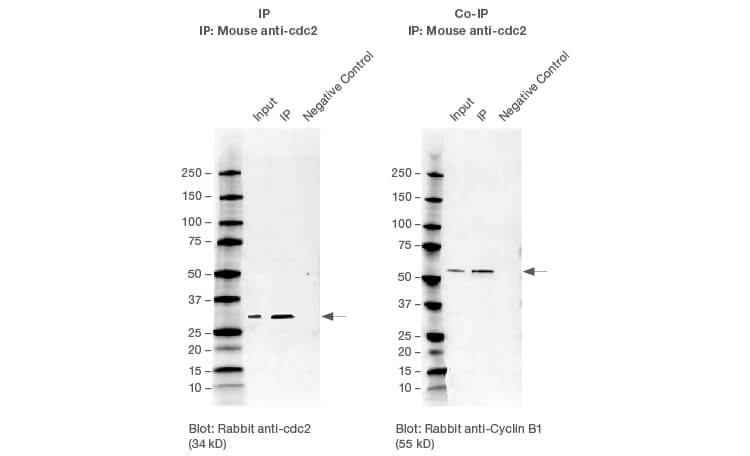
Successful IP and Co-IP results. HEK293 whole cell lysates were immunoprecipitated with cdc2 using the SureBeads IP protocol. Cyclin B1 was successfully co-immunoprecipitated with cdc2.
Protocol: Co-IP with SureBeads Magnetic Beads
Sample Preparation
- Determine the best cell line for target of interest.
- Grow at least 3.6 x 107 cells (two 80–95% confluent T-175 flasks).
- Split cultures 24–48 hr prior to harvest.
- Grow cells to mid-log growth (determine corresponding confluency or density).
- Lyse cells using this lysis buffer:
- 20 mM Tris (pH 7.5)
- 150 mM NaCl
- 1 mM EDTA
- 1 mM EGTA
- 1% Triton X-100
- Add fresh protease inhibitor before use
- IP freshly prepared lysates to avoid complex dissociation.
- IP using SureBeads Magnetic Beads.
Co-Immunoprecipitation
- Select SureBeads Protein A or Protein G Magnetic Beads appropriate for the antibody used for IP.
- Vortex SureBeads to resuspend them and transfer 100 µl (1 mg at 10 mg/ml) of SureBeads to tube.
- Magnetize beads and discard supernatant.
- Wash 3x with 1 ml PBS-T (1x PBS + 0.1% Tween-20).
- Add 7.5 µg of antibody. Rotate 20 min at room temperature.
- Wash 3x with 1 ml PBS-T.
- Add 1 mg of antigen-containing lysate. Rotate 1 hr at room temperature.
- Magnetize beads and wash 3x with 1 ml PBS-T.
- Elute with 20 µl of 2x Laemmli Buffer. Incubate for 5 min at 90°C.
- Magnetize and transfer supernatant to new vial (IP sample).
- Elute again with 20 µl of 2x Laemmli Buffer. Incubate for 5 min at 90°C.
- Magnetize and transfer supernatant to new vial (IP sample).
- Run SDS-PAGE.
See how others are using SureBeads Magnetic Beads to achieve experimental success.
Learn more about SureBeads Magnetic Beads.
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions. SureBeads are manufactured with magnetic bead technology from JSR Life Sciences. All trademarks used herein are the property of their respective owner.

