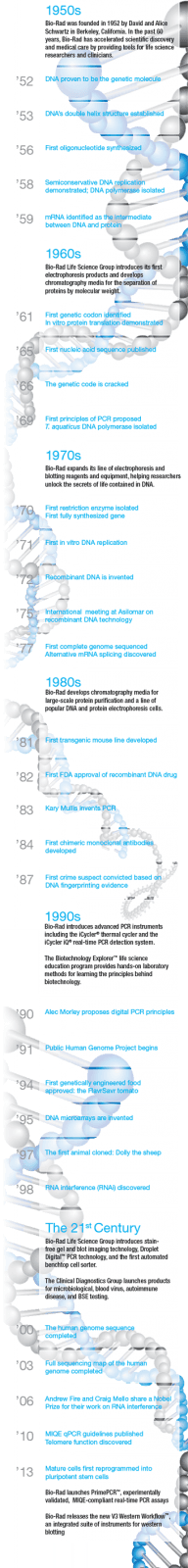
The discovery of the double-helix structure of DNA 60 years ago led to a revolution in biological science, opening the floodgates for myriad subsequent discoveries and spawning new fields of research. Bio-Rad has been there from the beginning, helping scientists, educators, and clinicians advance basic research and improve healthcare. As we celebrate Bio-Rad’s diamond anniversary, we reflect on the major events in the evolution of life science research, from biochemistry to molecular biology and beyond, and the emergence of modern biotechnology.
Although the origins of biotechnology arguably lie in prehistoric times with the advent of agriculture and fermentation, and the term “molecular biology” was originally coined in the late 1930s, the discoveries of the past six decades laid the foundations of modern molecular biology and biotechnology, giving rise to new technologies and industries that have changed the world in numerous ways, with applications in medicine, agriculture, and other fields.
By the 1950s, genetics was an established field; mechanisms of heredity had been deeply studied, and it was long suspected that deoxyribonucleic acid (DNA) was the molecule responsible for mediating genetic information. In 1952, Alfred Hershey and Martha Chase published experimental results that definitively confirmed DNA as the genetic carrier. Hershey and Chase would go on to win a Nobel Prize for this major finding and related research. However, it was a discovery published the following year that is widely acknowledged as the beginning of the genetics revolution.
In 1953, Nature published a landmark series of papers on DNA structure in 1953—by Watson and Crick; Wilkins, Stokes and Wilson; and Franklin and Gosling. The work of these researchers firmly established that DNA is a double helix with antiparallel nucleotide chains and specific base pairings. These insights led to great advances in biochemistry and gave birth to the new discipline of molecular biology. The elucidation of DNA’s structure enabled the cracking of the genetic code and provided keys to the solution of other long-standing mysteries of heredity and cellular function. An explosion of scientific research and discovery followed in the next 60 years.
The 1950s: A Decade of Discoveries
The 1950s was an exciting period for biochemists. Discoveries about the structure and function of biological macromolecules came in rapid succession after DNA was identified as the carrier of genetic information. Shortly after the watershed Nature publications, Alexander Todd achieved the first directed synthesis of a dinucleotide, Har-Gobind Khorana and colleagues pioneered phosphodiester oligonucleotide synthesis, and Francis Crick extended the nascent model now known as the central dogma of molecular biology by proposing that RNA acts as an intermediary between DNA and protein. Key to this model is the mechanism of semiconservative DNA replication, which was demonstrated by Matthew Meselson and Franklin Stahl in 1958. In the same year, Arthur Kornberg identified the key enzyme in the process, DNA polymerase, and first produced DNA in vitro. By the end of the decade, messenger RNA was positively identified as the intermediary between DNA and protein, confirming the central dogma.
The 1960s: Setting the Stage for a Revolution
The discoveries in biochemistry continued in the following decade. In vitro protein translation was demonstrated by Marshall Nirenberg and Heinrich Matthaei in 1961, and the first genetic codon was identified by Crick’s group. Building on Khorana’s work, groups led by Robert Letsinger and Colin Reese developed phosphotriester oligonucleotide synthesis methods. Robert Holley published the first nucleic acid sequence (tRNA), and the genetic code was cracked by Nirenberg, Holley, and Khorana, winning a 1968 Nobel Prize. With a potential not to be realized for years to come, Khorana and Kjell Kleppe proposed the first principles of the polymerase chain reaction (PCR), and Thomas Brock isolated a thermostable DNA polymerase from a hot spring bacterium in Yellowstone National Park
The 1970s: Modern Biotechnology is Born
The discoveries of the prior two decades served as a platform for advances in the 1970s that led to the development of biotechnology as we now know it: recombinant DNA, cloning, and gene synthesis and sequencing.
Hamilton Smith and others identified the first restriction enzyme and demonstrated sequence-specific DNA cutting, paving the way for the production of recombinant DNA and transgenic organisms. High-throughput commercialized DNA synthesis enabled the production of the first synthetic gene, and the first in vitro DNA replication, a precursor to PCR, was reported. The concept of recombinant DNA technique was jointly proposed by many researchers in 1972–1973. The 1974 U.S. patent for the first successful recombinant DNA application was awarded to Cohen and Boyer, who subsequently founded Genentech, considered by many to be the first true biotechnology company.
In 1977, Louise Chow, Richard Gelinas, Thomas Broker, and Richard Roberts at Cold Spring Harbor Laboratory published their amazing discovery of messenger RNA sequence rearrangement. In tandem with independent research by Phillip Sharp, Susan Berget, and Claire Moore at MIT, they developed a model of DNA that had gene sequences composed of coding exons and noncoding introns, with gene expression determined in part by alternative mRNA splicing; Roberts and Sharp shared a 1993 Nobel Prize for this work.
As those in the life science research community recognized the power and potential of recombinant DNA technology, they began an initiative for self-regulation, drafting ethical and safety guidelines for molecular biology and genetic research at a landmark international meeting in Asilomar, California. Edwin Southern developed the eponymous DNA blotting technique, which enabled researchers to identify, locate, and quantitate specific DNA sequences in a sample of genomic DNA, for example, to detect a genetically modified organism or to clone a native gene. By the end of the decade, Frederick Sanger had developed an efficient DNA sequencing method, and the first genome sequence of a DNA-based organism, bacteriophage fX174, was published.
The 1980s: A New Research Paradigm and the Commercialization of Biotechnology
In 1982, the FDA approved the first recombinant protein drug: insulin for the treatment of diabetes, developed by Genentech. The recombinant protein spared millions of diabetic patients from the risks associated with insulin extracted from cows or pigs.
In 1983, Kary Mullis invented the polymerase chain reaction, and the subsequent wave of PCR-based innovations opened entirely new research possibilities for countless scientists. Also in 1983, Barbara McClintock was belatedly recognized with a Nobel award for her discovery of transposons during the 1940s and 1950s; transposons are now widely used as valuable genetic research tools.
The first recombinant chimeric monoclonal antibodies were developed in 1984, giving rise to a host of modern therapeutics, and the DNA sequence of HIV was published in 1985.
In 1987, Susumu Tonegawa won a Nobel Prize for the elucidation of antibody production, laying the groundwork for a new generation of antibody-based diagnostic assays and therapeutic drugs. The first crime conviction based on DNA evidence was in the same year. Alec Jeffreys established the use of electrophoresis techniques to analyze DNA polymorphisms in forensics in 1989, coining the term “DNA fingerprinting.”
The 1990s: A Period of Consolidation and Expansion
The 1990s saw a wave of applications based on discoveries in the previous decades and the arrival of huge volumes of genomic and transcriptomic data from automated sequencing and DNA microarray technologies. The publicly funded Human Genome Project began in 1990, and gene therapy was tested on human patients for the first time. The yeast genome was completed in 1996, and the Caenorhabditis elegans genome was completed in 1998. A private effort to sequence the human genome did not start until 1998, but advances in the speed of DNA sequencing and data analysis allowed them to finish at the same time as the public project.
The invention of DNA microarrays by Pat Brown and colleagues revolutionized the analysis of gene expression patterns in an organism or population. Extending the basic principles utilized in Southern blotting, these high-resolution, genome-wide hybridization arrays led to the generation of huge volumes of expression data and the blossoming of a new discipline, genomics.
In concert with the rapid development of computing power and software sophistication, the new field of bioinformatics developed in response to the immense amounts of sequencing and microarray data being produced. The storage, annotation, and analysis of biological data rapidly began to generate insights of biological relevance, for example, the prediction of gene function from coding sequences, elucidation of noncoding DNA structures such as promoters and enhancers, and the relationships of genes involved in metabolic and signaling pathways, both in normal cells and in disease states such as cancer.
1997 saw two major milestones in biotechnology: the cloning of Dolly the sheep, and the first tests of gene therapy on human patients. Both were impressive technical achievements of controversial impact. The cloning of mammals is highly inefficient, and ethical considerations preclude cloning humans. However, cloning may be important in preserving endangered species or creating transgenic livestock breeds. After early failures and the death of several patients, some, including respected voices, urged a moratorium on gene therapy. However, more recent trials have been quite promising for the treatment of formerly intractable diseases such as leukemia and Parkinson’s disease or genetic disorders like severe combined immunodeficiency (SCID).
The 21st Century and Beyond: New Challenges, New Technologies
Genome sequencing proceeded at an accelerating pace in the next decade; in the year 2000, the Drosophila and Arabidopsis genomes were completed, and the human genome was fully completed in 2003. The year 2005 also saw the launch of the Genographic Project, a map of historical human migration patterns based on DNA samples from hundreds of thousands of people worldwide.
By the early years of the 21st century, quantitative real-time PCR (qPCR) had become a standard technique for the analysis of nucleic acids in applications such as forensics, food safety testing, and gene expression analysis. However, without careful validation of the targets, primers, and probes, as well as diligent extraction and amplification protocols and data analysis, qPCR assays are prone to error, a problem addressed by the MIQE guidelines (minimum information for publication of quantitative real-time PCR experiments; Bustin et al. 2009).
There are also fundamental physical limitations to the capabilities of qPCR assays, notably their reliance on relative quantitation with standards. In 1990, Alec Morley proposed the use of limiting dilution to realize a digital PCR method that could in principle deliver absolute quantification. Almost 20 years later, the principle was put into practice with the introduction of the first digital PCR systems, which use sample partitioning technology to provide absolute quantification of target DNA molecules with unrivaled precision, accuracy, and sensitivity without a standard curve. Novel applications were soon developed, leading to dozens of publications citing the new technique. Coming full circle, digital PCR pioneer Morley selected Bio-Rad’s QX100™ Droplet Digital™ PCR system to develop a new leukemia test in his lab.
Stem cell therapy holds great promise for the treatment of currently incurable conditions including blindness, deafness, paralysis, and Alzheimer’s disease. A major advance was the discovery that mature cells can be reprogrammed using only four well-known genes — Oct3/4, Sox2, c-Myc, and Klf4 — to become pluripotent stem cells, winning Shinya Yamanaka and John B. Gurdon a 2012 Nobel Prize. As stem cell therapy faced substantial early hurdles due to controversy over the source of fetal cell lines, the ability to use somatic cells as a source of new stem cells is key to realizing their therapeutic potential.
A Bright Future for Biotech and for Bio-Rad
Today, biotechnology promises to continue to change our lives for the better, providing cleaner, greener fuels, improved crops, and better medical treatments based on our increasing knowledge of cellular processes and the wealth of genomics and proteomics data available to researchers. In the near future, personal genome sequencing may make it possible to tailor diagnosis and treatment to each patient, and gene therapy has the potential to cure formerly incurable inherited diseases.
For the past 60 years, Bio-Rad has retained the entrepreneurial spirit of its early days while following its charter to accelerate scientific discovery processes by providing products and tools for life science researchers. Bio-Rad now employs approximately 7,600 people worldwide, serving over 100,000 academic and industrial customers with a broad range of products and services for researchers and clinicians. Bio-Rad continues to innovate and plays a leading role in the advancement of scientific discovery, helping people live longer, healthier lives.
For more information about our company’s history, mission, or products and services, visit us at www.bio-rad.com.



