Ever gotten lost and had to find your way back? How do we know where we are while venturing into a new space? What is that “déjà vu” moment we experience when we navigate our way into a previously visited space? It’s all in the brain, as they say.
This year’s Nobel Prize in Medicine was awarded to three neuroscientists, John O’Keefe, May-Britt Moser, and Edvard I. Moser for their discovery and characterization of brain cells that are responsible for navigation. The last time a Nobel Prize was awarded for brain research was in 1949 to Walter Hess for his work on mapping and localizing the parts of the brain that are responsible for various autonomic functions of the body. Sixty-five years later, the work that earned this most recent Nobel Prize now allows us to understand how individual cells in the brain lay out a map to let us understand where we are in space and how to negotiate our ways through known and unknown territories. In short, these neuroscientists have shown that there is a global positioning system (GPS) in the brain.
How does this so-called inner or brain positioning system work? How are we integrating the information about already known locations when we move into a new space? Does the brain have a map? Is it like a cartographic map with information only about landmarks? Or a cognitive map with topological data that allow us to make meaningful connections between the landmarks? Fortunately for us, rats provide a lot of answers to these questions.
How Does a Rat Know Its Place?
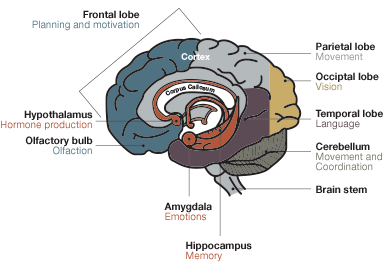
The idea that our brains might have a spatial map of our environment was proposed long ago (Tolman 1948). But it wasn’t until the discovery of special brain cells called place cells (O’Keefe and Dastrovsky 1971) that the idea of the brain’s GPS was found to be a reality. O’Keefe took a neurophysiological approach and in his experiments he inserted an electrode into a rat’s brain and recorded the activity of the neurons in that particular brain area. His experiments revealed that every time a rat crossed a specific landmark known to the animal, particular cells located in the hippocampus (see Figure 1 for location of hypothalamus) fired. He called this special group of cells place cells. The place cells were shown to generate firing fields (areas with concentrated firing activity) each time the animal crossed the specific spot, which he called a place field (Figure 2).
Cross Section of the Brain
 Place Cells of the Hippocampus
Place Cells of the Hippocampus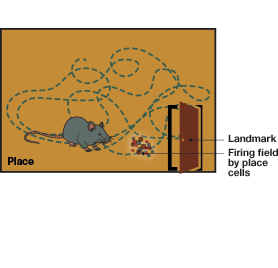
Fig.1. Cross section of the brain. The hippocampus is situated in the temporal lobe, underneath the cerebral cortex. It is associated with memory and spatial navigation. Removal of the entire hippocampus results in complete loss of memory.Fig.2. Place cells of the hippocampus. Place cells fire exclusively when an animal crosses specific landmarks. John O’Keefe, one of the Nobel laureates of 2014, discovered these cells in 1971. Place cells are part of the brain’s GPS, providing location information.
Place cell firing is influenced by external sensory information, like landmarks, and this information is integrated with the information about the animal’s position. O’Keefe proposed that a spatial map exists in the brain and that this map is fine-tuned and updated as it receives more information about its environment (O’Keefe and Nadel 1978). All the nerve firings were observed in the hippocampus, and so he suggested that the hippocampus carries the spatial map.
So, Can the Place Cells Process All Spatial Information?
In reality, is this all the information we need to understand the spatial map? How do we integrate the information of space, distance, and one’s own position and apply it all in order to navigate our way through a three-dimensional space? Two decades later, this question was still an intriguing one for neuroscientists. It was clear that there was more processing done in the brain than merely understanding one’s place in space. Another clue emerged from the work of Taube et al. in 1990, with the discovery of a new type of cells in the subiculum area connecting the more distal entorhinal cortex to the hippocampus (See Figure 3 for the anatomy of the hippocampus).
A.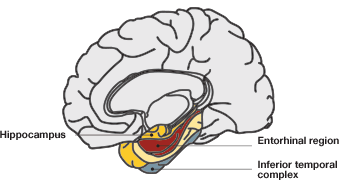 B.
B.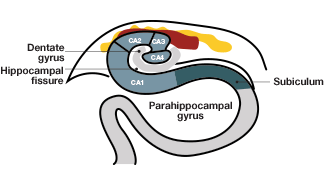
Fig.3. Hippocampal anatomy. A, the hippocampal and parahippocampal regions. The entorhinal cortex, indicated in red, provides nervous input into the hippocampus; B, anatomy of the hippocampal and entorhinal cortical regions. The CA3 region of the entorhinal cortex has direct input into the CA1 region of the hippocampus, providing spatial information.
Head Direction Cells Follow the Movement of the Head
Taube et al. (1990 A, B) found that a specific group of cells, which they named head-direction cells, fired when a rat moved its head in a certain direction (Figure 4). This neuronal activity was independent of the animal’s location or behavior — or even of the position of its own trunk! In addition, the firing of these cells corresponded to specific manipulations in the rats’ environment (such as rotating a cue card that was placed in the containers to provide spatial cues for orientation purposes), suggesting that head-direction cell firing enables the animal determine its spatial relationship with its environment. The results of their experiments showed that the head-direction cells could guide the animal’s directionality as well as improve its spatial problem-solving ability by providing information about external coordinates. The firing of the head-direction cells is one-dimensional, based only on the direction of the head’s movement and independent of all other parameters. Thus, these cells differed from the place cells, whose firing is two-dimensional, determined by both the X and Y coordinates of the animal’s position (taking into account where the animal is and what landmark it is crossing). The head-direction cells help the animal navigate by providing clues about directionality.
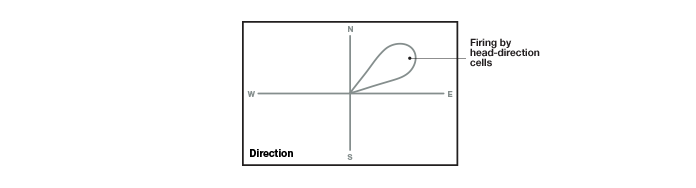
Fig.4. Head-direction cells. These cells in the subiculum fire when a rat moves its head in a certain direction. The firing occurs independently of the animal’s location or behavior and of the direction of the rest of the body. Head-direction cells provide a sense of direction to the animal.
Integrating Information about the Third Dimension
To unravel the mysteries of a possible three-dimensional mental map in the brain, May-Britt Moser and Edvard Moser traversed the entire hippocampal area with their electrodes. By injecting ibutenic acid and lesioning different portions of hippocampus in the rat brain, the Mosers were able to show that cells responsible for spatial memory are distributed across different regions of the hippocampus, encoding different functions (such as spatial memory retrieval vs. ability to learn about new places and integrate new information). Thus, they predicted the existence of a widespread neural network in the hippocampus, rather than a localized area, for processing spatial information (Moser and Moser 1998). With the same experiments, they found that the dorsal side of the hippocampus was more important for memory retrieval and surmised that signals could be coming from adjacent areas of the hippocampus, dorsal to the hippocampal CA1 region (see Figure 3).
Where Exactly Is the Signal Coming From?
The next step, then, was to establish which cells in the dorsal side transmit this signal to the hippocampus. A study by Brun et al. (2002) provided an answer. They removed all input into the hippocampus, leaving intact only that from the entorhinal cortex, a small portion adjacent to the hippocampus, and found that all spatial memory–associated tasks remained completely normal. Thus, they established that the circuitry between the entorhinal cortex and the hippocampus is sufficient to maintain all basic tasks associated with spatial memory in the hippocampus. Subsequently, they further narrowed the signaling area down to the dorsomedial part of the entorhinal cortex (dMEC) as the probable source for providing spatial signals to the hippocampus (Fyhn et al. 2004). This was the breakthrough the Mosers were waiting for. Being able to focus on this small area proved more advantageous for deciphering the finer circuits in the brain’s GPS than focusing on the entire hippocampal area.
Rats Map Out Where They Are Going for Food (or Dessert)
A seminal study by the Mosers in 2005 established that the little-known entorhinal cortex carried a neural map that the rats referred to while picking up food (in this case, pieces of chocolate) scattered in a defined space (Hafting et al. 2005). By inserting electrodes into the dMEC, the Mosers measured the nerve firing of individual neurons in this area. What they observed was an amazing array of concentrated firing fields in the animal’s path, just like the specific firing fields of place cells in the hippocampus (Figure 5).
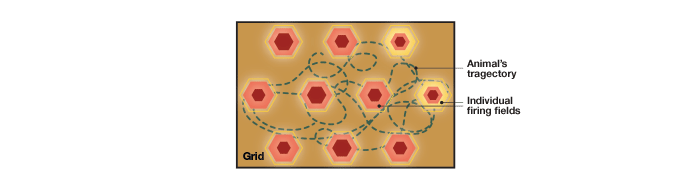
Fig. 5. Grid cells. These cells in the medial entorhinal cortex produce a firing pattern like a grid, with the firing fields forming the pattern of repeated (tessellating) triangles. The pattern is repeated across the rat’s trajectory and is uniform across all firing fields. All grid cells in the medial entorhinal area produce similar grid-like firing patterns. Grid cells provide latitudinal and longitudinal information for the animal.
In contrast to the place cell firing fields that are specific to one particular location, the Mosers found several firing fields throughout the animal’s path, following some pattern not easily deciphered. Expanding the space in which the rats could move aided in discerning the pattern of the firing fields. Each firing field followed a triangular pattern that was repeated over and over again (a tessellating triangular pattern). The Mosers hypothesized that each firing unit (which they named a grid cell) forms a grid-like firing pattern with the geometry of a triangle.
A.
 B.
B.
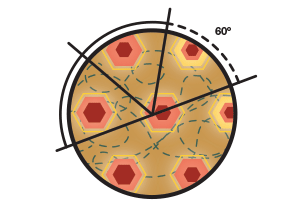
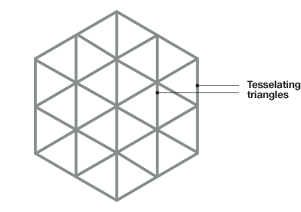
Fig. 6. The tessellating triangles form the shape of a hexagon. A, The hexagonal shape formed when the triangular pattern is repeated over and over again. B, The near constant angular separation from the central peak to the sixth peak (always close to 60°).
The tessellating trianglular pattern in each firing field would then yield the shape of a hexagon (Figure 6). The firing patterns (spikes of firing activity) were analyzed by looking to see if the multiple firing fields of the same neuronal cell indeed formed a regular structure (spatial autocorrelation ), and if there is any correlation between pairs of cells recorded at the same time (spatial crosscorrelation). From these they derived autocorrelograms and crosscorrelograms for the firing data. For each autocorrelogram, they compared the distance and angle from the central peak of nerve firing to the surrounding peaks in the firing field. Because of the repeating triangular pattern, they predicted that in each firing unit the central peak should be surrounded by six peaks, and the distance between the central peak and the sixth peak would be constant, thus forming the vertices of a regular hexagon.
This was indeed the case. In an extended space, all these individual hexagonal patterns were repeated across the rat’s path, equidistant from each other and with an equal angular separation between them (60° within one hexagon and multiples of 60° between the hexagons; Figure 6B). The Mosers uncovered a remarkable algorithm that the brain was using to compute the most efficient way to negotiate space!
Is the Grid Map Based on Internal or External Cues?
In order to find out if the map gathers information from cues provided by external landmarks (allothetic cues) or from the rat’s own memories (idiothetic cues), the Mosers changed the local environment (for example, by rotating the cue card in the container by 90°) and checked whether the nerve firing was realigned to that modification. The grid pattern did indeed closely align with the changes in landmarks in the rats’ environment, supporting the idea that the phase and orientation of the grid is set by external landmarks. In addition, the Mosers also found that the grid activity persisted in darkness and after the removal of cues. Together these data suggested that grid activity is independent of all allothetic information, but allothetic cues are important for aligning the grid to an external reference frame. They also found that the grid cells formed new firing field patterns in a novel environment (like mapping out a new place). Thus the map is not a cartographic map, but consists of topological information about the surroundings that is constantly updated.
Cells of the Same Grid Pattern Talk Together
Another interesting and important finding by the Mosers is that all the grid cells in that dMEC area showed a similar grid-like firing pattern with similar spacing and field size between them. The grid cells in that neighborhood all show an identical hexagonal firing pattern (albeit of different sizes), with their phase (the vertex location in the hexagon) so close that the neighboring grids can all be nearly superimposed upon each other. The size of the pattern changed with the distance of these cells from the entorhinal border.
Negotiating Edges and Peripheries
In addition to finding place (location), direction, and reference points, we also need to negotiate borders and edges. The Mosers’ further studies on rats show that there are specialized cells, called border cells, which endow this ability (Solstad et al. 2008). These are a small population of cells, which fire only when a rat hits the borders of its container, and serve as reference points for the animal’s environment by defining a perimeter within which all the cells needed for providing spatial information processing exert their function (Figure 7).
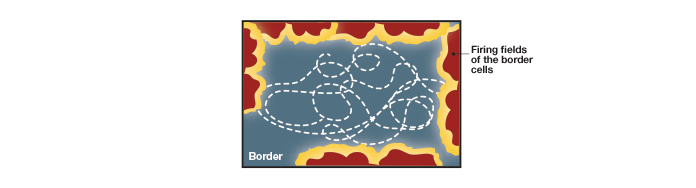
Fig.7. Firing fields of the border cells. These specialized cells in the medial entorhinal cortex fire when the animal is close to a border or periphery of the container. In experiments, the firing pattern persisted even when the shape of the containers was changed or when artificial borders were introduced within the container. Border cells define a perimeter for all the cells providing spatial information.
Integrating It All in the Brain’s GPS
So how do we collate all this information together to form an integrated map of space and keep it updated constantly? So far, our understanding is that the brain’s GPS consists of these four known components with known functions: the place cells, the head-direction cells, the grid cells, and the border cells, all functioning as an ensemble. The neural circuitry between these cells has been established. The signal from place cells provides an updated sense of our location, the head-direction cells help establish directionality, the border cells provide the perimeter of a given space, and the grid cells offer a sense of latitude and longitude. Together, these cells create the brain’s GPS module as we understand it now (Figure 8). With changes in the environment, new and updated information is overlaid in space by the grid cells, forming memories.
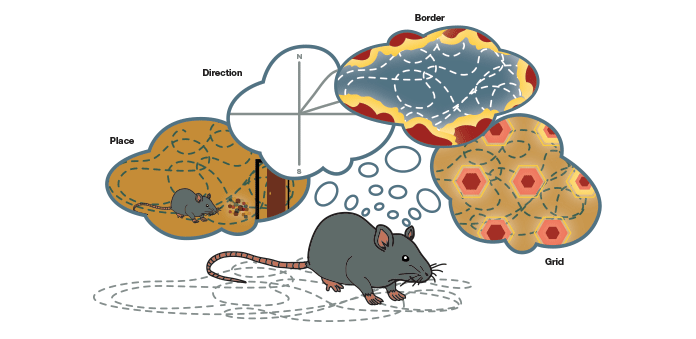
Fig.8. The brain’s GPS. The constituents of this system coordinate their firing to enable navigation in a three-dimensional space. The signal from place cells provides information on location, the head-direction cells help with directionality, the border cells provide the perimeter of a given space, and the grid cells give a sense of latitude and longitude.
In addition to these four types, other cells have also been identified in the entorhinal cortex whose roles are yet to be determined. The ability of the brain to navigate in space depends on the coordinated effort of all these cells put together.
It is amazing to see how the brain orchestrates these individual components into a coordinated movement in three-dimensional space. Our understanding of our own ability to navigate in space has been greatly strengthened by these findings. Given that the identification of the players, their functions, and how they are coordinated, have all been deciphered by John O’Keefe, Edvard Moser, and May-Britt Moser, the choice of this year’s Nobel Prize winners is a no-brainer!
References
Brun VH (2002). Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 296, 2243–2246.
Fyhn M (2004). Spatial representation in the entorhinal cortex. Science 305, 1258–1264.
Hafting T et al. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806.
Moser MB and Moser EI (1998). Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci 18, 7535–7542.
O’Keefe J and Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34, 171–175.
O’Keefe J and Nadel L (1978). The Hippocampus as a Cognitive Map (Oxford: Clarendon Press).
Solstad T et al. (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868.
Taube JS et al. (1990). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10, 420–435.
Taube JS et al. (1990). Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10, 436–447.
Tolman EC (1948). Cognitive maps in rats and men. Psychol Rev 55, 189–208.

