Understanding the peaks is the key to a successful chromatography experiment.
Here are some basic definitions of all the terms related to peaks, what you can determine from the FPLC peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins.
Anatomy of the peak
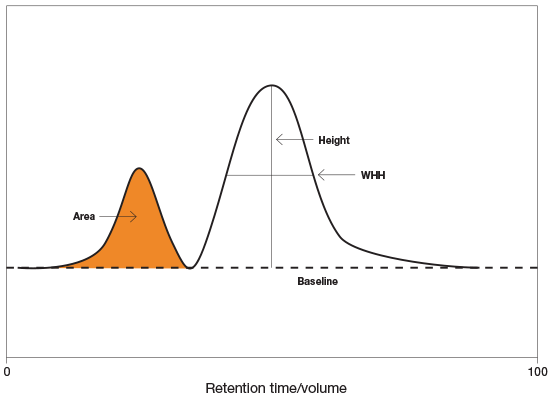
In general, the AS is a measure of the quality of column packing. Well-packed columns will have an AS of close to 1. All measurements are made at 10% of the maximum peak height.
Purity measured by peak area will only take into account purity levels against proteins that absorb at the wavelength used for detection.

