By 2020 the worldwide market for biologics is expected to exceed $390 billion (up from $46 billion in 2002) and account for 28% of the global pharmaceutical market. As they are developed across a wider range of therapeutic areas, biosimilars will play a more important role by providing a greater choice of treatment options (Aitken 2016).
Worldwide access to biologics is a real issue due to their exorbitant cost. In developing countries with limited resources that primarily focus on fatal diseases, there may not be coverage for biologics for their population. In more than 50% of European countries the annual cost of a biologic exceeds their per capita GDP by up to 11 times.
It is hoped that the development of biosimilars will lead to more competition, and therefore, more accessible, cost-effective treatments. While there are challenges in making biosimilars, the timeline for their development is approximately five years less than that for an originator biologic. Biosimilars can offer cost savings from 10–30% over biologics and their availability allows better access to newer therapies (Lorenzetti 2015).
Current Opportunity for Biosimilars
Several high-priced biologics (worth $81 billion) are approaching patent expiration, providing an opportunity for manufacturers to develop more affordable biosimilars. The eight top-selling biologics losing patent exclusivity across Western Europe and the U.S., an equivalent of $56 billion, focus on two major therapy areas, inflammation and diabetes, and include adalimumab and enteracept for rheumatoid arthritis (RA), Crohns’ disease, psoriasis, and other inflammatory diseases and insulin glargine for type I and II diabetes.
Case Study: The Global Impact of RA
About 1% of the global population is affected by RA. It primarily afflicts women, mostly between the ages of 30 and 55, during their most productive adult years. It’s a chronic condition in which the immune system attacks the lining of the joints causing swelling, stiffness, and pain. If RA is left untreated, it can lead to irreparable joint damage (Figure 1). Up to 40% of RA patients stop working within five years of diagnosis due to increasing disability. In addition to the constant pain, fatigue, and deformity, patients are at greater risk of developing cardiovascular disease, infections, and mental health disorders. The risk of mortality is also double that in the normal healthy population (American College of Rheumatology 2017). Fortunately, there are some encouraging advances in therapeutic treatment for RA patients.
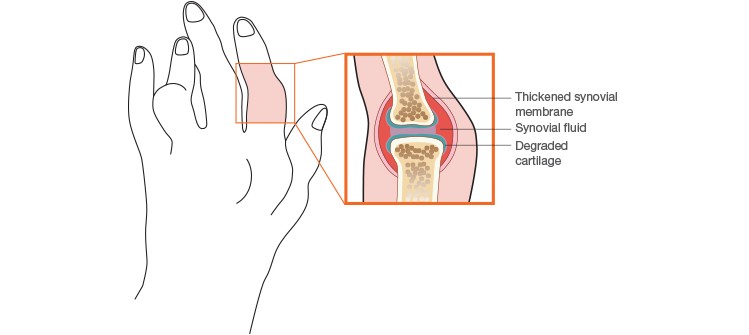
Fig. 1. Effect of rheumatoid arthritis on a joint.
Biosimilars in RA Treatment
Fifteen years ago, the treatment objective for RA was to balance the deterioration from poorly controlled disease with the long-term side effects of corticosteroid use. In recent years, the introduction of biologics such as rituximab, adalimumab, and etanercept have revolutionized RA care (Table 1). Between 30 to 70% of patients who do not benefit from first-line medications experience some measure of relief from biologics. Roughly 40% of patients don’t respond to the first biologic they try and switch to another biologic (Consumer Reports 2013). While these biologics are used to treat symptoms, they are not a cure. In addition, they are very expensive, costing in excess of $5,000 per week, and cause their own side effects. Some fortunate RA patients have achieved remission on a biologic and have even been able to sustain it after drug reduction or cessation. Although biologics are not a perfect solution for RA patients, their adoption has marked a huge improvement in outcomes. However, more options are needed and the introduction and continued research in biosimilars could provide these options for patients.
Table 1. Therapeutics available for rheumatic diseases.
| Type | Originator Biologic | Biosimilars | Composition | Delivery | Side Effects |
|
Tumor Necrosis Factor (TNF) inhibitor |
Etanercept | CHS-0214 GP2015 HD203 SB4 |
Fusion protein, the IgG antibody is fused to a TNF inhibitor | Self-injection | Serious infections |
| TNF inhibitor | Adalimumab | ABP 501 BI 695501 CHS-1420 GP-2017 M923 SB5 ZRC-3197 |
Human monoclonal antibody (mAb) | Self-injection | Serious infections |
| TNF inhibitor | Infliximab | BOW015 CT-P13 PF-06438179 SB2 |
Part human, part mouse mAb | IV administered for 2 hr, 3 times/week for 6 weeks, then every 8 weeks | Serious infections |
| Interleukin-6 (IL-6) blocker | Sarilumab | N/A | Human mAb | Injection every 2 weeks | Infection, increase in liver enzymes |
| IL-6 blocker | Tocilizumab | N/A | Human mAb | IV administered over 1 hr, once/month | Infection, increase in liver enzymes |
| B-cell targeter | Rituximab | CT-P10 GP2013 PF-05280586 |
Part human, part mouse mAb | Two 4–6 hr infusions, 2 weeks apart, every 6–12 months | Infection, infusion reaction consisting of itching, rash, wheezing |
| IL-1 blocker | Anakinra | N/A | Man-made form of IL-1 receptor antagonist | Self-injected once/day | Infection |
| Janus kinase inhibitor | Tofacitinib | N/A | Small molecule drug | Oral | Increased liver enzymes, cholesterol, risk of shingles |
Data from Arthritis Foundation 2017 and Dorner et al. 2016.
Currently, over 20 biosimilars targeted to the RA patient population have become available on the market in more than 70 countries. In addition, the pipeline has over 700 biosimilars in preclinical and clinical trial development for use in rheumatic diseases (Dorner et al. 2016).
Continuing Clinical Concerns
In order to obtain regulatory approval, the FDA, EMA, and other regulatory bodies require a pharmacokinetics/pharmacodynamics (PK/PD) study and at least one randomized clinical trial (RCT) to show efficacy, immunogenicity, and comparable safety of the biosimilar when compared to its originator biologic. The confirmation RCT needs to be designed using an equivalence margin comparing primary endpoint differences between active treatment and placebo statistically derived from a meta-analysis of previous RCTs with the originator (Cohen and Kay 2017).
Once a biosimilar is approved for use, there are some relevant clinical concerns when transitioning from the originator biologic. There are no publically available studies that discuss manufacturing changes in the originator biologic although posttranslational differences do occur. Most RCTs studying transitions to a biosimilar include only one randomized switch after assessment of the primary endpoint. More than a decade of experience in the European Union has demonstrated that transitioning from the originator filgrastim to its biosimilar has not lowered efficacy or increased adverse events. However, prescribers are still wary of transitioning patients from one therapeutic to another without additional data. Two studies, NOR-SWITCH (sponsored by the Norwegian government) and PLANETAS (extended from the original PK/PD study of infliximab in ankylosing spondylitis) are currently examining the issue of transitioning. RA patients will continue to be important test subjects for biosimilars because they allow researchers to obtain insights into immunogenicity by measuring anti-drug antibodies.
What Happens Next?
Biosimilars have now entered clinical practices globally for the treatment of RA and other diseases. Limited experience, mostly in the form of RCTs comparing one product to placebo, indicates that biosimilars have similar efficacy and safety profiles to their corresponding originator biologics. Harmonizing regulatory guidelines and recommendations for use will reduce concerns and increase usage. If the cost savings of biosimilars are realized, worldwide accessibility will increase and usage will rise. This will provide patients worldwide with RA, other autoimmune conditions, and many other diseases a cost-effective improvement in treatment options and enhanced quality of life.
References
Aitken M (2016). Delivering on the potential of biosimilar medicines: The role of functioning competitive markets. iqvia.com/-/media/iqvia/pdfs/institute-reports/delivering-on-the-potential-of-biosimilar-medicines.pdf, accessed December 27, 2017.
American College of Rheumatology (2017). Rheumatoid Arthritis. rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis, accessed December 27, 2017.
Arthritis Foundation (2017). Biologics overview. arthritis.org/living-with-arthritis/treatments/medication/drug-types/biologics/drug-guide-biologics.php, accessed December 27, 2017.
Cohen S and Kay J (2017). Biosimilars: Implications for rheumatoid arthritis therapy. Curr Opin Rheumatol 29, 260–268.
Consumer Reports (2013). Using biologic drugs to treat rheumatoid arthritis: Comparing effectiveness, safety, side effects, and price. consumerreports.org/cro/2013/03/using-biologic-drugs-to-treat-rheumatoid-arthritis/index.htm, accessed December 27, 2017.
Dorner T et al. (2016). The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 75, 974–982.
Lorenzetti L (2015). Biosimilars may one day save your life. But what are they? fortune.com/2015/02/06/biosimilars-what-are-they/, accessed December 27, 2017.
Arthritis Foundation is a trademark of The Arthritis Foundation, Inc.

