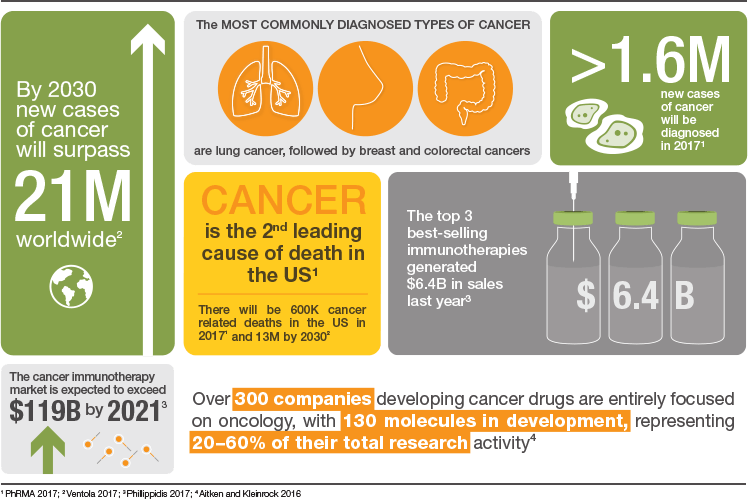
Every year cancer kills eight million people worldwide. By 2030, the number of annually diagnosed cases will exceed 21.7 million resulting in 13 million cancer deaths due to aging and population growth.
Lung, breast, colorectal, and prostate cancer are the four most commonly diagnosed cancers with lung cancer taking the lead (Ventola 2017). Although enormous progress has been made, treatments still include surgery, radiation, and chemotherapy with many patients either not responding well or relapsing. In addition to the treatments, patients must also manage a wide variety of undesirable side effects that impact their quality of life.
While it has been previously established that the immune system can recognize cancer cells, it is also known that cancer cells are very good at evading its normal surveillance functions. Over the past decade, advances in oncology research have resulted in many new treatment options that include immunotherapies. Active therapies that induce an immune response include cytokines, monoclonal antibodies (mAbs), and cancer vaccines. Passive therapies that stimulate a patient’s intrinsic immune response include: bispecific T-cell engagers (BiTE Antibodies), bispecific and multispecific antibodies, oncolytic viruses, cell-based therapies, and tumor-targeting mAbs (Morrissey 2016).
Let’s examine a couple of the newer therapies in more detail.
Using Malignant Cells to Target the Immune System
Learn about Cancer Immunotherapy Development
Get InfoAn interesting and innovative technology called BiTE is being developed at Amgen to engage the body’s endogenous T-cells to target malignant cells. Ordinarily activated T-cells will use various mechanisms to eliminate target cells causing cytotoxic components to be released. Malignant cells have the capability to evade destruction by cytotoxic T-cells and BiTE technology is designed to overcome malignant cell evasion by binding polyclonal cytotoxic T-cells and targeted malignant cells. Its clinical effectiveness is currently being investigated.
Vaccines and Cancer
Cancer vaccines can be either preventative or therapeutic. As the former, they are used to prevent cancer from developing in healthy people, such as the preventative vaccine for cervical cancer that guards against strains of human papillomavirus that are known to cause the disease. As the latter, they are meant to be used as a treatment to strengthen the body’s immune response, for example, the vaccine available for prostate cancer.
Another new process called Lm Technology, designed to activate the immune system by using the body’s natural ability to recognize and attack bacterial infections, is being developed by Advaxis Immunotherapies. This technology alters a live, attenuated strain of Listeria monocytogenes to stimulate cancer-fighting T-cells to attack against cancer antigens and reduce tumor defense mechanisms, in turn enabling the immune system to destroy cancer cells.
Clinical Trial and Error
With ongoing improvements in understanding the role of the immune system, huge advances have been made in immunotherapy. However, this does not mean that every immunotherapy trial is successful. In July 2016, Juno Therapeutics had to stop a trial of genetically engineered white blood cells, a CAR-T therapy that was being used to treat adult lymphoblastic leukemia patients, when three patients died. The incident was used to regroup and closely assess the safety and potency of combination therapies and served to remind everyone that biology can be tricky.
The Robust Pipeline of Immunotherapy Drugs
From 2005 to 2015, the pipeline of oncology drugs in clinical development expanded 63%, with 87% of the new development concentrated on targeted therapies that included protein kinase inhibitors and mAbs as well as other mechanisms that could identify or block cancer cell multiplication. From 2011 to 2015, seventy new oncology treatments were launched and are being used to treat more than 20 tumor types, among them leukemia, lung cancer, multiple myeloma, melanoma, and lymphoma. It is expected that many of these compounds will be approved for additional indications. The newest available immunotherapy drugs use PD-1, PD-L1, and CTLA-4 mechanisms to assist the patient’s immune system to attack cancer cells (Aitken and Kleinrock 2016).
A Diverse Set of Companies Developing Immunotherapies
More than 500 companies are actively developing cancer-focused drugs, most commonly for non-small cell lung cancer and breast, prostate, ovarian, and colorectal cancers. The majority of these companies have pipelines entirely focused on oncology indications each with between one to seven compounds in development.
The immunotherapy market is expected to grow to almost $120 billion by 2021 (Phillippidis 2017). A large number of companies are collaborating with one another in order to develop these new therapies. The top three collaborations are listed in Table 1.
Table 1. Collaborations of companies co-developing new immunotherapies.
| Companies | Deal Value | Date | Objectives |
|
Merck & Co. and Ablynx |
$6.4 billion | 22 July 2015 | Develop up to 12 cancer drugs based on antibody fragments or Nanobodies |
| Bristol-Myers Squibb Company and CytomX Therapeutics | $2.9 billion | 20 March 2017 | Discover up to 8 targets (6 oncology) with a CTLA-4 Probody therapeutic |
| Pfizer, Cellectis, and Servier | $2.9 billion | 18 June 2014 | Develop CAR-T therapy for 15 targets selected by Pfizer |
Data from Phillippidis 2017.
Amgen currently has, in various stages of clinical development, the largest number (273) of potential immunotherapies (Figure 1). Merck & Co. (136), AstraZeneca (64), and AdaptImmune Therapeutics (59) follow at a distance. In total, the top 25 companies are developing 909 immunotherapies; 422 are in phase I/II clinical trials, 273 are in phase II/III, and 146 are in stage IV. Most of the immunotherapies in development are focused on proteins/antibodies (57) followed closely with a focus on T-cells (31) (Figure 2).
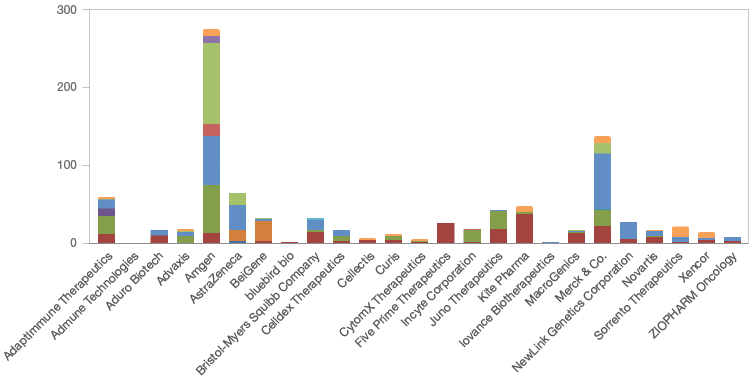
Fig. 1. The number and clinical trial stages of immunotherapies being developed by the top 25 immunotherapy companies. Clinical trial stage 0 (■); I (■); I/II (■); I/IIa (■); I/III (■); Ib (■); II (■); II/III (■); III (■); IV (■); market (■); preclinical (■).
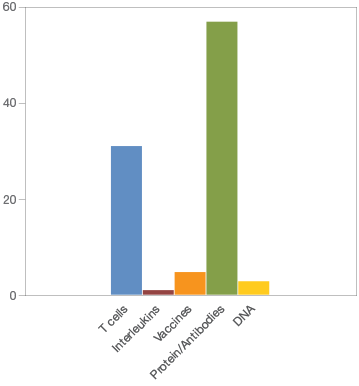
Fig. 2. Focus of immunotherapies currently in development.
What Happens Next?
Cancer is one of the most lethal diseases globally. Much progress has been made in developing immunotherapies and their rapid development and promising initial success play a role in current cancer treatments. Different treatment strategies have elicited varying outcomes in anti-tumor effect. Going forward, it will be important to identify patients that will benefit from therapy through the use of predictive biomarkers. Effective combination therapies as well as the timing or duration of immunotherapy administration will also be future challenges to overcome. The future looks bright as research continues into multiple strategies and agents.
Learn about what’s new in immunotherapy and how it is becoming the next frontier for cancer.
References
Aitken M and Kleinrock M (2016). Global oncology trend report: a review of 2015 and outlook to 2020. imshealth.com/en/thought-leadership/quintilesims-institute/reports/global-oncology-trend-report-a-review-of-2015-and-outlook-to-2020, accessed September 6, 2017.
PhRMA (2017). Medicines in development for immuno-oncology 2017 report. phrma.org/medicines-in-development-immuno-oncology, accessed October 27, 2017.
Morrissey KM et al. (2016). Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin Transl Sci 9, 89–104.
Philippidis A (2017). Top 10 immuno-oncology collaborations. genengnews.com/the-lists/top-10-immuno-oncology-collaborations/77900887, accessed October 19, 2017.
Ventola CL (2017). Cancer immunotherapy, part 1: current strategies and agents, P T 42, 375–383.
BiTE is a trademark of Amgen Inc. Lm Technology is a trademark of Advaxis Immunotherapies. Probody is a trademark of CytomX Therapeutics, Inc. Nanobodies is a trademark of Ablynx N.V.

