Abstract
RT-qPCR is a well-established method for the detection, quantification, and typing of different microbial agents in the areas of clinical and veterinary diagnostics, as well as food safety. There are multiple benefits of this technology: unparalleled sensitivity, quick time to results, simultaneous detection of multiple targets, scalable throughput, and low cost. However, when dealing with the challenges of pathogen detection, not all RT-qPCR reagents are created equal. In this application note, we demonstrate a sensitive codetection of viral RNA and DNA targets in a multiplex setting using a novel one-step multiplex RT-qPCR supermix.
Figures
Introduction
Bio-Rad’s Reliance One-Step Multiplex Supermix is a reagent that enables researchers to sensitively and accurately detect up to five targets in a single reaction. This mix is based on a novel enzyme, Reliance Reverse Transcriptase — a chimeric enzyme derived from FLV (feline leukemia virus) and MMLV (Moloney murine leukemia virus) reverse transcriptases. Reliance Reverse Transcriptase, in combination with a powerful blend of DNA polymerases and an expert formulation, gives the mix unparalleled robustness and stability. As a consequence of this, reaction plates prepared with the Reliance One-Step Multiplex Supermix can be held at room temperature for an extended period of time with no loss in target detection sensitivity.
Materials and Methods
We designed an experiment testing samples with three viral nucleic acids, two in their RNA form — Zika (ZIKV) and chikungunya (CHIKV) — and the DNA version of dengue virus (DENV), with an internal positive control (IPC) RNA and human genomic DNA (gDNA) control. The viral targets were tested at five different dilutions while the controls were kept constant. The copy numbers for the viral RNA targets were determined by real-time Droplet Digital PCR and were diluted to a single copy or less per reaction in their lowest dilution series (Table 1). We made two identical plates, each containing ten technical replicates of each sample; one plate was analyzed immediately (time (T) = 0 hr), while the second plate was stored at room temperature (25°C) and analyzed the next day (T = 24 hr).
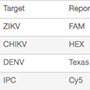
Table 1. Targets and templates used for a 5-plex reaction.
| Target | Reporter Dye | Template | Amount | RNA Copies |
| ZIKV | FAM | Viral RNA | 5 dilution series | 1.17 to 11,668 |
| CHIKV | HEX | Viral RNA | 5 dilution series | 0.15 to 1,498 |
| DENV | Texas Red | Synthetic DNA | 5 dilution series | N/A |
| IPC | Cy5 | Synthetic RNA | Constant | N/A |
| RNaseP | Cy5.5 | Human gDNA | Constant | N/A |
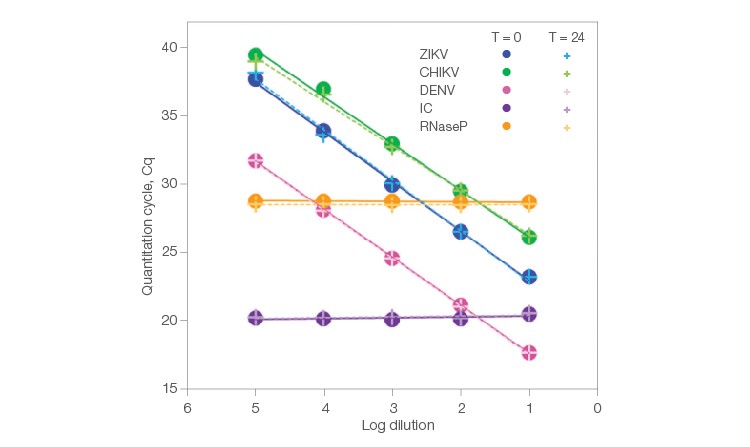
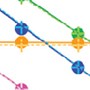
Fig. 1. Codetection of various nucleic acid targets in 5-plex reaction.
Results and Discussion
Room Temperature Stability of Reliance One-Step Multiplex Supermix
As shown in Figure 1, we observe remarkable consistency between the data produced at T = 0 hr (solid circles) and T = 24 hr (crosses). The T = 0 hr and T = 24 hr points generally overlap, indicating that the amount of target quantified by Reliance One-Step Multiplex Supermix is stable after holding the preassembled reactions at room temperature for one day. The detected amount of viral nucleic acid over the dilution series changes as expected with excellent efficiency and R2 values (Table 2). All viruses were detected in all five sample dilutions at both time points (Table 2).
When working with fewer copies of RNA (one or less), not all technical replicates of a sample are expected to be positive. For example, based on a Poisson distribution, a sample that has, on average, one RNA copy per reaction should lead to 6–7 positive reactions out of 10 tested. This type of distribution is observed in ZIKV dilution 5 where 1.17 RNA copies are expected in each technical replicate (Table 3). Here, seven out of ten technical replicates are positive at T = 0 hr and T = 24 hr, aligning perfectly with expectations. Similarly, CHIKV is detected at both time points in a technical replicate series where an average of only 0.15 RNA copies is expected (Table 3). These results imply that Reliance One-Step Multiplex Supermix enables detection of single RNA copies at close to 100% efficiency, even in reactions that have been prepared 24 hours earlier.
Comparison to Other Reagents
We also analyzed one-step multiplex reagents offered by other companies. At T = 0 hr, both companies’ products detect the viruses (Tables 2 and 3). However, Company A had poor efficiency amplifying CHIKV and only detected 3 of the 5 CHIKV dilutions and 4 of the 5 ZIKV dilutions. Company B performed better than Company A, yet still missed detecting CHIKV is sample dilution 5 (Table 3). At T = 24 hr, however, the performance of both companies’ reagents degraded significantly (Tables 2 and 3). This is a clear demonstration of the uniqueness of Reliance One-Step Multiplex Supermix for its ability to retain sensitivity and efficiency even after 24 hours at room temperature. This feature is critical when screening large numbers of samples, since high-throughput workflows involve the testing of multiple plates. Adopting a reagent which is stable at room temperature would circumvent the need for refrigerating plates, and therefore enable much simpler automated solutions.
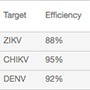
Table 2. Comparison of 5-plex detection at two time points.
| T = 0 hr | T = 24 hr | ||||||
| Reagent | Target | Efficiency | R2 | Dilutions Detected (Out of 5) |
Efficiency | R2 | Dilutions Detected (Out of 5) |
| Reliance One-Step Multiplex Supermix |
ZIKV | 88% | 0.999 | 5 | 86% | 0.996 | 5 |
| CHIKV | 95% | 0.996 | 5 | 102% | 0.996 | 5 | |
| DENV | 92% | 0.999 | 5 | 92% | 0.999 | 5 | |
| Company A | ZIKV | 82% | 0.991 | 4 | N/A | N/A | 1 |
| CHIKV | 68% | 0.990 | 3 | N/A | N/A | 0 | |
| DENV | 107% | 0.999 | 5 | 79% | 0.987 | 3 | |
| Company B | ZIKV | 81% | 0.998 | 5 | 82% | 1.000 | 2 |
| CHIKV | 84% | 0.996 | 4 | N/A | N/A | 1 | |
| DENV | 96% | 0.999 | 5 | 72% | 0.959 | 4 | |
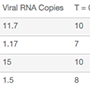
Table 3. Comparison of a number of ZIKV and CHIKV replicates detected at two time points over 24 hours.
| Reliance One-Step Multiplex Supermix |
Company A | Company B | ||||||
| Viral Target | Log dilution | Viral RNA Copies | T = 0 hr | T = 24 hr | T = 0 hr | T = 24 hr | T = 0 hr | T = 24 hr |
| ZIKV | 4 | 11.7 | 10 | 10 | 10 | 0 | 10 | 0 |
| ZIKV | 5 | 1.17 | 7 | 7 | 0 | 0 | 5 | 0 |
| CHIKV | 3 | 15 | 10 | 10 | 9 | 0 | 10 | 0 |
| CHIKV | 4 | 1.5 | 8 | 9 | 0 | 0 | 7 | 0 |
| CHIKV | 5 | 0.15 | 2 | 4 | 0 | 0 | 0 | 0 |
Conclusion
Reliance One-Step Multiplex Supermix can detect and quantify five targets simultaneously with outstanding efficiency and single-copy detection sensitivity. Additionally, the supermix can codetect RNA and DNA targets effectively, allowing for the development of panels detecting various types of pathogens (e.g., RNA viruses, DNA viruses, bacteria, yeast). Finally, this reagent’s performance is not affected by extended storage of assembled reactions at room temperature thereby enabling simpler and more reproducible automated workflows.
These results highlight the fact that Reliance One-Step Multiplex Supermix can be used easily in high-throughput screening workflows and applications that require high-sensitivity detection, in particular for pathogen detection. Combining Reliance One-Step Multiplex Supermix with Bio‑Rad’s CFX Automation System II (a robotic plate handler) and two CFX Real-Time PCR Systems would allow for large numbers of plates (up to 40, depending on the cycling protocol) to be screened for up to five RNA or DNA targets — with no operator assistance.
Learn more about our Reliance One-Step Multiplex Supermix.