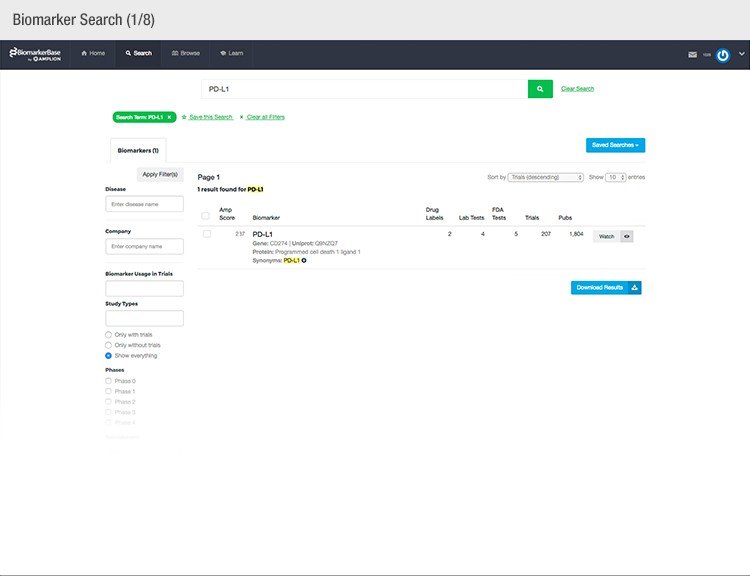
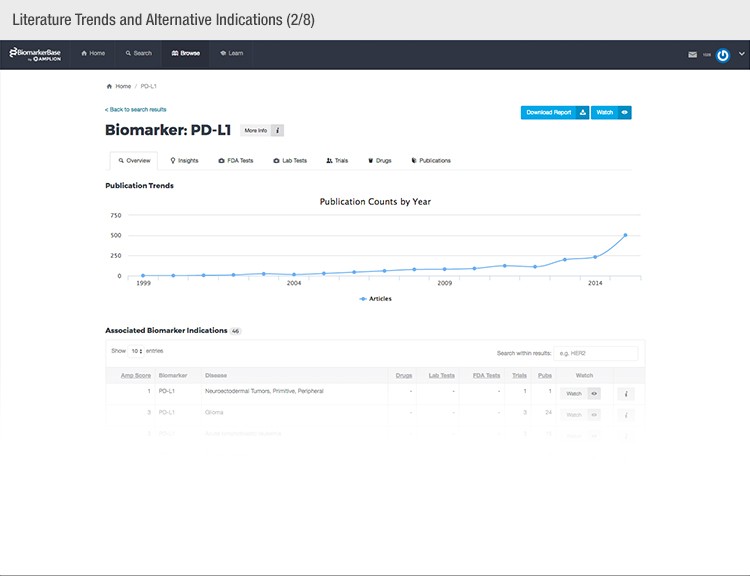
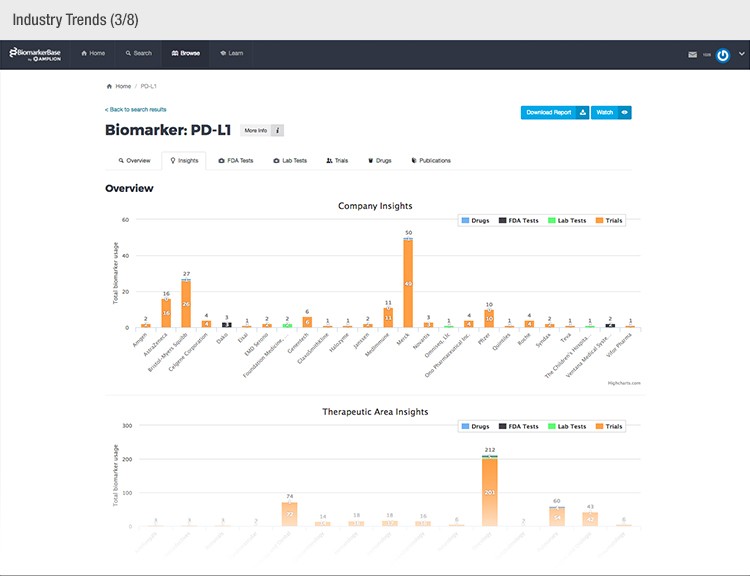
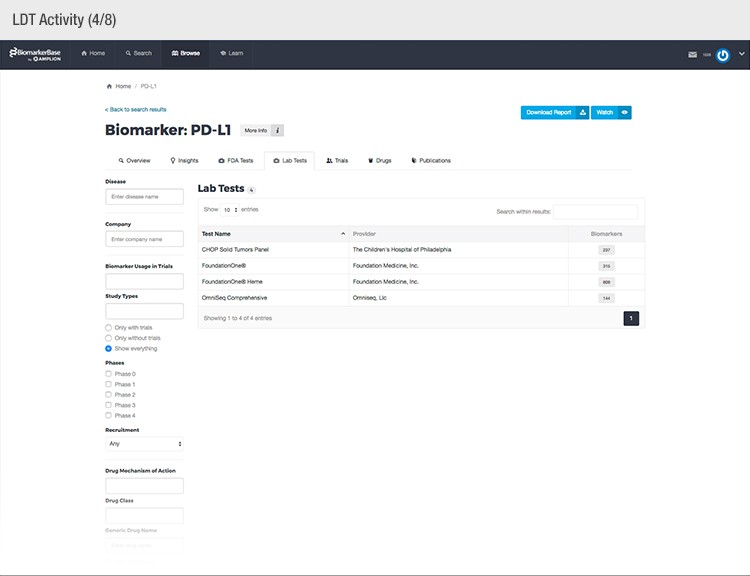
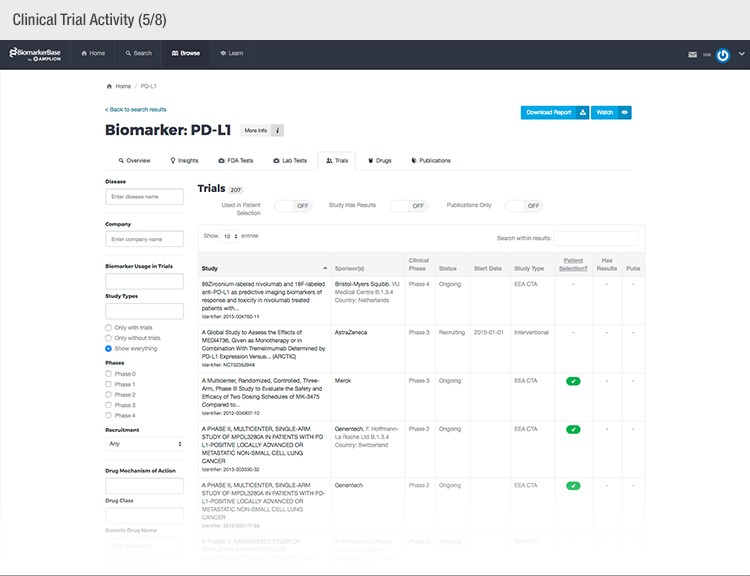
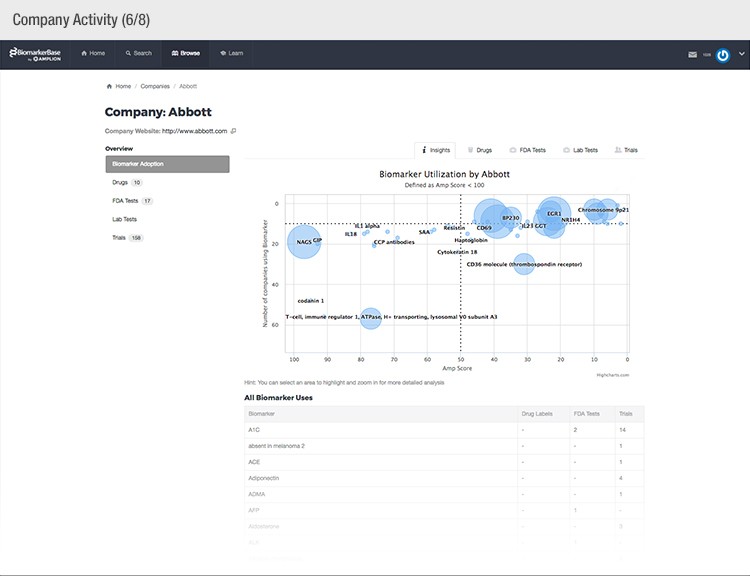
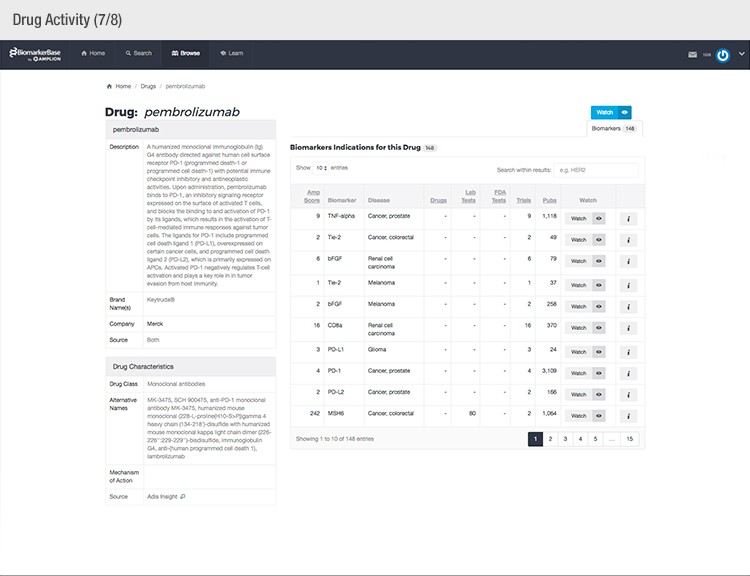
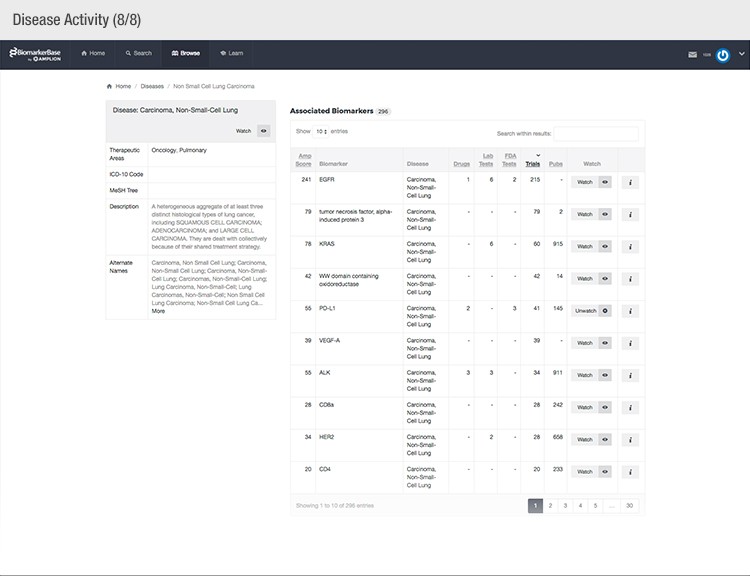
The typical success rate of a clinical trial is less than ten percent. While a single trial can cost upwards of $50 million, there is a strong sense of urgency to implement methods that reduce costs and increase a drug’s Likelihood of Approval (LOA).
Pharmaceutical companies are shifting to Electronic Data Capture (EDC), adopting earlier Phase II end-points, and even conducting trials in other countries in an effort to lower costs. Through early collaboration with regulatory agencies and use of Clinical Research Organizations (CROs), companies are also cutting trial timelines. Ultimately though, therapeutic efficacy and the safety of trial participants are the decisive factors for regulatory approval.
With recent advances in precision medicine, more and more companies are using biomarker screening to identify and select the patients most likely to respond to specific therapies and exclude those prone to significant side effects. By only enrolling patients who pass the biomarker screen, the drug is more likely to work effectively on trial participants, leading to more success. In fact, the use of these patient selection biomarkers was proven to triple the LOA from 8 percent to an astonishing 24 percent in a recent clinical development study published by Amplion Inc, Biotechnology Innovation Organization (BIO), and BioMedTracker (Thomas 2016). The challenge now is to identify the most relevant biomarkers for a given disease and drug and then develop tests to evaluate them.
Incorporating Patient Selection Biomarkers
Biomarkers are measurable biological signatures, such as genes or proteins, indicative of a particular disease state or response to therapy. Diagnostic and prognostic biomarkers diagnose patients and indicate how a disease may develop. Predictive biomarkers, also known as efficacy and safety biomarkers, anticipate which patients will benefit from a medication, and which will have potentially severe side effects. The use of predictive biomarkers is critical to precision medicine, which relies on patient stratification to define which patients should receive treatment.
The combination of precision medicine and well-correlated predictive biomarkers ensures that the treatment will be most effective and least toxic for a well-defined group of patients. Before the medication can even be prescribed, however, it must gain regulatory approval via the completion a successful clinical trial. Predictive biomarkers can, and should, be incorporated early in the trial to maximize the LOA by selecting for patients most likely to receive a benefit from the therapeutic.
To choose the most predictive biomarkers, biomarker strategy should be considered early in the drug development cycle. However, choosing the right biomarker to complement a drug requires a comprehensive understanding of disease pathways and the biomarker landscape, that is, biomarker intelligence (Figure 1).

Fig. 1. Biomarker attributes of interest. Listed are some of the information, characteristics, features that need to be collected and understood before a biomarker is selected for a clinical trial.
“Pharmaceutical companies need a comprehensive picture of what’s already known about a particular biomarker, how others have used it, what’s been published on it, and if it’s been used in other clinical trials,” explained Adam Carroll, Chief Scientific Officer at Amplion. “It’s also important for them to know if there’s already an FDA-approved or laboratory-developed test for it, or if a test is in development.”
Finding the Answer in an Ocean of Data
The answers to these questions can be found if you can navigate the ever-growing ocean of biomarker data. It is virtually impossible to do a manual search through the many data sources to gain true biomarker intelligence. Time is of the essence when every day that passes before going to market eats into the patent lifespan of the drug costing the pharma/biotech company billions of dollars in lost revenue.
“The challenge that we took on at Amplion was to make it easier to get a look at the whole landscape for a particular biomarker or disease area, at a glance,” said Carroll. “ We’re focused on taking the universe of biomarker information and digesting it on behalf of the industry so that they can make use of it to better plan their clinical trials. By pulling together diverse sources of information and putting them in one place, pharmaceutical companies can watch trends develop to make knowledgeable business decisions.”
To achieve their mission, Amplion has developed an intuitive biomarker knowledgebase named BiomarkerBase powered by the Amplion Data Acquisition Platform (ADAP), a proprietary artificial intelligence (AI) algorithm used to rapidly and accurately collect and curate biomarker data (Figure 2). The ADAP resolves information about biomarkers from FDA cleared tests, Lab Developed Tests (LDTs), clinical trial data from the U.S. and E.U., drug labels, and peer-reviewed publications. With two publications added to PubMed every minute, data curation relies heavily on automation. Machine learning algorithms are developed and trained on a set of data annotated by Amplion scientists dictating what are valid or invalid data. Once trained, the AI is able to automatically categorize and make connections among the variably sourced biomarker data, consistently incorporating new data into BiomarkerBase.
Fig. 2. Vast amounts of knowledge can be found in the BiomarkerBase tool.
“We rely on a machine learning technique called active learning,” said Carroll, who is responsible for the scientific review and quality assurance of data processing and development of algorithms and maintenance of the AI. “In active learning, when the AI algorithms generate a result, they also generate a confidence. We can then look at the decisions made by the algorithm that are the least confident and address the predictions by adding new data to the training set and continuously improve the algorithms.”
The constant improvement of the ADAP provides a reliable source of biomarker data that is organized and analyzed within BiomarkerBase. The platform links biomarkers with drug targets, diseases, drugs, therapeutic areas, and companies while providing specifics and trends on past and present clinical trials, publications, and available tests for each biomarker. Users can rapidly gain an understanding of the biomarker landscape to make strategic decisions that may make or break their clinical trial.
Continual Biomarker Surveillance
The information found in BiomarkerBase is valuable throughout the entire lifecycle of drug development. However, early strategic evaluation is critical, and there can be adverse consequences if this is not performed or thoroughly investigated. Starting a trial without a biomarker and introducing it later is problematic, as the therapeutic and corresponding biomarker are not co-developed. Several phases of the trial would need to be repeated to demonstrate that the biomarker is a proper indicator of therapeutic response, safety, or patient outcome. This results in longer development time and higher costs.
Over the course of a decade long trial, a great deal of new data will be generated for any given biomarker, which may initiate a shift in thinking. Companies can find it valuable to continually survey the landscape and monitor changes from the start of the trial. BiomarkerBase allows users to watch biomarkers, alerting them to the latest information about biomarkers of interest. This feature ensures that current information about a co-developed biomarker is known when taking a drug to market and that the most effective commercialization program is assembled.
While biomarker intelligence will assist the pharmaceutical industry in advancing novel therapeutics to market, there are other sectors that also benefit from a comprehensive view of the biomarker landscape. Diagnostic companies use BiomarkerBase for competitive analyses and market assessments to determine if it is worth adding a new test to their repertoire. Life science companies track trends to inform the direction of a particular disease area or biomarker.
Adding Amplion’s tool to their clinical trial management is one of many ways that pharma is becoming smarter and faster in developing therapies. “There’s an enormous amount to be learned from the information within BiomarkerBase,” proclaimed Carroll, whose main motivation is to help improve human health. “We’re in a great position to help patients. It’s a great time to be alive!”
References
Thomas DW et al. (2016). Clinical development success rates 2006–2015. http://www.amplion.com/clinical-development-success-rates, accessed May 2, 2017.
BiomarkerBase is a trademark of Amplion Inc. BioMedTracker is part of the Business Intelligence Division of Informa PLC.