What is the definition of child abuse? To neuropathologist Dr. Bennet Omalu, it is allowing children to play football. In an interview with ESPN writer Kevin Seifert last August, Dr. Omalu said, “I’ve always said that every child who plays football has a 100 percent risk of exposure to brain damage” (Seifert 2017).
Linking Football to Degenerative Brain Disease and Mental Illness
Dr. Omalu knows firsthand how abusive football can be to the brain. In 2002, he discovered chronic traumatic encephalopathy (CTE) in a football player. CTE is caused by repetitive insults to the brain, resulting in diffuse accumulation of amyloid plaques throughout the brain. These plaque deposits are also seen in patients with Alzheimer’s disease. In fact, many of the symptoms of CTE overlap with Alzheimer’s disease.
Patients suffering from CTE may experience the following symptoms, which can take up to 10 years to manifest (McKee et al. 2013):
- Cognitive impairment
- Impulsive behavior
- Depression or apathy
- Short-term memory loss
- Loss of executive function
- Emotional instability
- Increased substance abuse
- Suicidal thoughts and behavior

The player at the center of Dr. Omalu’s discovery was Mike “Iron Mike” Webster who played center for the Kansas City Chiefs and Pittsburgh Steelers from 1974 to 1990. After retirement, Webster suffered from amnesia, dementia, and depression. Dr. Omalu’s discovery of the connection between football and Webster’s severe mental illness, as well as the national football league’s (NFL’s) subsequent denial of that finding, was portrayed in the 2015 film Concussion.
In 2005, Dr. Omalu published his findings based on Webster’s case in the journal Neurosurgery in a paper titled “Chronic Traumatic Encephalopathy in a National Football League Player” (Omalu et al. 2005).
Dr. Omalu’s research sparked enormous controversy in the NFL. In 2006, the league’s traumatic brain injury (TBI) committee requested the retraction of Dr. Omalu’s paper, citing the research as “completely wrong” and “a failure.” Despite the pushback, Dr. Omalu continued researching CTE in football players and published another study in 2006, which showed CTE in the brain of Terry Long, a former NFL player who suffered from depression and committed suicide in 2005 (Omalu et al. 2006).
This second study was also presented to the league and was again met with suspicion and ultimately complete denial. The NFL’s TBI committee chair, Ira Casson, told the press: “In my opinion, the only scientifically valid evidence of CTE in athletes is in boxers and in some Steeplechase jockeys” (Ezell 2013). It wasn’t until 2009 that the NFL finally acknowledged a possible link between football and CTE.

Since that acknowledgement, the league has continued to witness the tragedies of suicides in multiple players with confirmed CTE: Junior Seau in 2012, who shot himself in the heart to preserve his brain for research, and Aaron Hernandez, who was sentenced to life in prison and later hung himself in his jail cell. Hernandez’s brain revealed the worst CTE ever discovered for someone his age. Other players who have taken their own lives since 2009 include Shane Dronett, Dave Duerson, Ray Easterling, Jovan Belcher, and Paul Oliver — every one of them with evidence of CTE.
A 2017 study in the Journal of the American Medical Association (JAMA) revealed that 110 out of 111 deceased former NFL players who donated their brains to research had developed CTE. It is important to note that CTE is not the only brain injury that can result from trauma to the brain. Commenting on this study, Dr. Omalu said, “There has been so much fascination with CTE that we are going the wrong way. CTE is just one disease in a spectrum of many diseases caused by brain trauma. If he doesn’t have CTE, that doesn’t mean he doesn’t have brain damage” (Seifert 2017).
Previously, CTE could be confirmed in the brain only during an autopsy. In November 2017, a study published in Neurosurgery revealed the first living patient to have confirmed CTE using brain scans (Omalu et al. 2017). The study describes the brain scans of 14 former NFL players. The scans revealed a distinctive pattern of tau buildup in the brain, which is unique to CTE patients. Upon the death of one of those players, an autopsy was performed to confirm the diagnosis. Although further research needs to be done to confirm the predictive validity of the brain scans for CTE diagnosis, this is a promising step toward achieving earlier diagnoses.
TBI — Beyond CTE and Football
The established link between football and brain damage is truly tragic. However, there is a sport that has an even higher incidence of TBI than football — girls’ soccer. A recent study led by Dr. Wellington Hsu, professor of orthopedics at Northwestern University’s Feinberg School of Medicine, reported that 27% of all injuries in girls’ soccer are TBI-related (compared to 24% in football) (Schallmo et al. 2017). The researchers noted, “To our knowledge, this is the first study to report that concussions now account for a higher proportion of injuries in girls’ soccer than boys’ football. The concussion rate for girls’ soccer is also increasing rapidly and is now nearly tied with boys’ football and is threefold higher than boys’ soccer.”
The Today Show interviewed Dr. Omalu regarding the risk of TBI and various sports. When asked about soccer he said, “Soccer is a high-dexterity, high visual-spatial coordination sport. You need very high levels of brain functioning to play it and children have not attained that level of brain development. Soccer, as it’s played today, should be played only by children who are above the age of 12–14.” Dr. Omalu also recommends eliminating heading the ball until age 18 (Pawlowski 2017).
According to the Centers for Disease Control, there were 2.5 million reported TBIs in the United States in 2010, resulting in direct and indirect costs of approximately $76.5 billion.
TBI Progression and Severity
TBI occurs in two phases: the primary and secondary injuries. The primary injury occurs at the moment of head trauma, causing deformation of blood vessels, axons, glia, and neurons. This primary injury is irreversible and preventive measures such as helmets and airbags should be employed. Secondary injury is potentially reversible and occurs over the course of hours and days following the initial insult. It involves a cascade of biochemical and physiological responses to the primary injury that can lead to severe brain damage and death (Plummer et al. 2016).

According to the Centers for Disease Control, there were 2.5 million reported TBIs in the United States in 2010, resulting in direct and indirect costs of approximately $76.5 billion. The severity of a TBI may range from mild (a brief change in mental status or consciousness) to severe (an extended period of unconsciousness or memory loss after the injury). A non-fatal severe TBI may result in an extended period of unconsciousness (coma) or amnesia after the injury. For individuals hospitalized after a TBI, almost half (43%) have a related disability one year after the injury.
A TBI may lead to a wide range of short or long-term issues affecting:
- Cognitive function (for example, attention and memory)
- Motor function (for example, extremity weakness, impaired coordination and balance)
- Sensation (for example, hearing, vision, impaired perception and touch)
- Emotion (for example, depression, anxiety, aggression, impulse control, personality changes)
Approximately 5.3 million Americans are living with a TBI-related disability, and the consequences of severe TBI can affect all aspects of an individual’s life. This can include relationships with family and friends, as well as their ability to work or be employed, do household tasks, drive, or participate in other activities of daily living (Bey and Ostick 2009).
Mild traumatic brain injury (mTBI) accounts for more than 75% of all TBIs (McInnes et al. 2017). Along with impaired cognitive function, mTBI causes an array of symptoms that include headaches, fatigue, depression, anxiety, and irritability, collectively referred to as post-concussion syndrome (PCS). It takes approximately three months for symptoms to resolve in the majority of individuals. However, some patients continue to experience symptoms beyond the first year after the injury. Those with such long-lasting symptoms are said to experience persistent PCS, which is estimated to impact 15% of individuals with a first-time concussion.
mTBI produces more subtle deficits, which can lead a patient or caregiver to underestimate the seriousness of the injury and return to an activity that may lead to continued exposure to further risk of mTBI. Frequent repetitive TBI, such as that which occurs over the course of a football player’s career, leads to CTE. However, repetitive mTBI can result in a much more acute, severe outcome. Repetitive mTBI that occurs within hours, days, or weeks (second impact syndrome or SIS) can cause diffuse cerebral swelling, brain herniation, and even death. SIS can occur with any two events involving head trauma. While rare, it is devastating, since young, healthy patients can die within a few minutes (McInnes et al. 2017).
TBI Diagnosis
Neuroimaging (brain scan) in the form of computed tomography (CT) is considered the gold standard of TBI diagnosis. A CT scan of the brain provides detailed information about damaged brain structures, including disruption of the blood brain barrier. Another scanning technology, magnetic resonance imaging (MRI), reveals changes in brain activity associated with trauma. However, neither CT nor MRI is practical for use in the field or on a repetitive basis due to low accessibility, high cost, and radiation exposure.
Diagnosing the severity of the initial insult as early as possible is critical in preparing for the downstream effects of a secondary injury. The only field-based assessment used currently is the Glasgow Coma Scale (GCS), which yields a score by assessing the eye movement as well as the verbal and motor response of the patient. The limitations of the GCS are that it takes a skilled practitioner to accurately perform the assessment, it is subjective (poor inter-assessor reliability), and lacks sensitivity when a patient is under the influence of drugs, alcohol, or stress (factors often related to TBI outside of sports). This can lead to underestimating the severity of the injury, thus risking inappropriate follow-up and treatment.
The lack of TBI follow-up can result in “talk and die” syndrome, a term used to describe the rapid deterioration of a head trauma patient, typically due to epidural hematoma (bleeding in the brain). This was the ultimate cause of the death of 45 year-old Natasha Richardson, actress and wife of actor Liam Neeson in March 2009. Richardson fell and hit her head while skiing on a beginner run at Mont Tremblant in Quebec, Canada. After her fall on a Monday morning, the ski patrol attended to her, but it is reported that she refused a doctor or ambulance. She returned to her room at the resort, and was monitored by a ski instructor and the manager of the hotel. Later that afternoon, her condition deteriorated and an ambulance was called to take her to the hospital. Unfortunately, by the time she arrived, doctors could not stabilize her. By Wednesday evening of the same week, Richardson was dead. Had the severity of her injury been detected on the mountain, she would have been rushed to the hospital to relieve the pressure from the bleeding in her brain, giving her a chance at survival.
Biomarkers in TBI
The current methods of TBI diagnosis are inadequate, especially given how common and debilitating the impact of the disease can be. Researchers have been striving for a method of TBI detection that is portable, openly accessible, and requires little training and expertise to administer. Experts in the field recognize that serum-based biomarkers represent the holy grail of potential TBI detection and prognosis (Figure 1, Table 1).
Unlike other diseases where specific biomarkers that provide a definitive diagnosis or severity for a disease have been identified (for example, HBA1c for diabetes), no such diagnostic biomarkers exist for TBI. For years, researchers have studied dozens of potential biomarkers that show promise, yet today there is still no FDA-approved biomarker available for clinical use. While blood-based biomarkers afford optimal convenience and accessibility in the field, serum biomarker alterations may not completely reflect the biochemical changes in the injured brain. This is because other factors, such as the integrity of the blood-brain barrier and the release of biomarkers from other injured organ systems, can confound what is specifically happening in the brain.
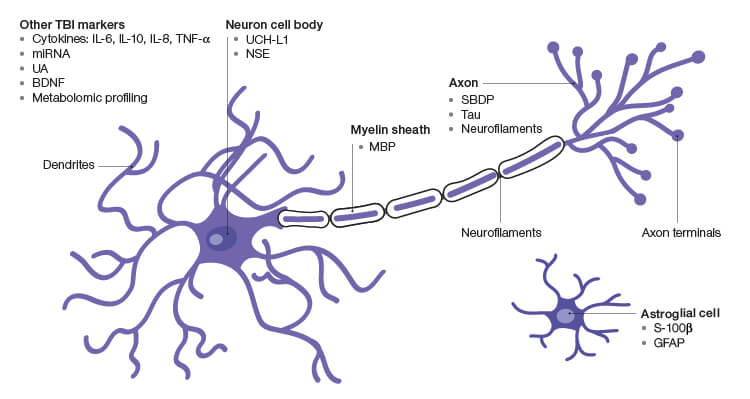
Fig. 1. Prospective TBI Biomarkers. BDNF, brain-derived neurotrophic factor; GFAP, glial brillary acidic protein; MBP, myelin basic protein; miRNA, microRNA; NSE, γ-enolase; SBDP, spectrin breakdown products; UA, uric acid; UCH-L1, ubiquitin C-terminal hydrolase.
Cerebrospinal fluid (CSF) biomarkers have the advantage of increased specificity over peripheral blood markers due to their increased proximity to the brain and decreased susceptibility to the confounding effects of factors outside the brain. However, the CSF resides in a closed system around the brain and spinal cord, making it impractical for sampling in the field.
Biomarker research is itself difficult to perform. A researcher in an academic or corporate setting may focus on a single marker within a cascade of biological activity that is likely dependent on upstream and downstream markers outside of their area of focus. Helping to solve this problem is a company called Amplion, which has built a biomarker search tool called BiomarkerBase, which uses machine learning to immediately deliver a comprehensive survey of the clinical biomarker landscape.
Table 1. Potential TBI biomarkers.
| IL-6, IL-8, IL-10 | The levels of interleukin-6 (IL-6), IL-8, and IL-10 are increased in CSF in response to severe TBI. The magnitude of the increase correlates with patient outcome and blood brain barrier (BBB) dysfunction, as indicated by the CSF: serum albumin ratio. However, these markers are not brain specific. They may be released into the peripheral tissues and slowly seep into the CSF (Neselius et al. 2012). |
| TNF-α | TNF-α is produced in the brain in response to various pathological processes such as infectious agents (for example, human immunodeficiency virus (HIV) and malaria), ischemia, and trauma (Feuerstein et al. 1994). |
| Ubiquitin C-terminal hydrolase (UCH-L1) | UCH-L1 is an abundant enzyme in the soma of neurons that cleaves ubiquitin. Its levels correlate with the severity of TBI and patient outcome in some studies, but the results are inconsistent in mTBIs. One study reported that serum UCH-L1 levels can discriminate mTBIs from controls, whereas some studies were unable to show a sufficient discriminating power between patients with mTBI and non-injured controls (Adrian et al. 2016). |
| γ-enolase (NSE) | In humans, early studies showed serum NSE to be elevated in mTBI patients although subsequent reports indicate a lack of clinical relevance despite elevated levels (Kulbe and Geddes 2016). |
| Spectrin breakdown products (SBDP) | Upon cellular injury, calpains and caspases (enzymes) cleave spectrin, a cytoskeletal protein that maintains cell membrane integrity and cytoskeleton structure, to spectrin breakdown products (SBDPs). SBDPs have been reported in the CSF early after injury as well as in adults with severe TBI. SBDP levels significantly correlate with the severity of injury and clinical outcome (Papa et al. 2015). |
| Tau | After TBI, tau is proteolytically cleaved (c-tau) and gains access to the CSF and serum. CSF levels of c-tau are significantly elevated in TBI patients, which correlate with clinical outcome. Total tau protein may be indicative of axonal damage in gray matter neurons and has been found to be associated with the scale of injury in severe TBI (Papa et al. 2015). |
| Neurofilaments (NF-L, NF-H) | CSF measurements of NF-L are regarded as the most sensitive markers of axonal injury. Phosphorylated NF-H has been found to be elevated in the CSF of adult patients with severe TBI (Papa et al. 2015). |
| Myelin basic protein (MBP) | Increases in CSF MBP have been reported following an array of neurological insults including TBI in adults and children (Su et al. 2012). |
| S-100β | S-100β helps to regulate intracellular levels of calcium in astrocytes and is considered a marker of astrocyte injury or death. Because S-100β is also found in non-neural cells such as adipocytes, chondrocytes, and melanoma cells, its correlation with TBI can be confounded by release from extracerebral tissues (Thelin et al. 2017). |
| Glial fibrillary acidic protein (GFAP) | After brain damage, GFAP is released from degenerating brain cells into the surrounding interstitial fluid and appears in the peripheral blood, likely through disruptions in the BBB. Elevated serum GFAP can provide diagnostic and prognostic information in a range of neurological diseases including severe TBI (Lei et al. 2015). |
| microRNAs | miRNAs can be detected in serum and can be an indicator of disease pathology in the cell of origin, including neuronal cells. Serum miRNAs are relatively stable at variable pH conditions and are resistant to repeated freeze/thaw cycles and enzymatic degradation, which make them a suitable biomarker candidate for TBI (Di Pietro et al. 2017). |
| Metabolomics profile | The metabolic profile provides a window into the in vivo enzymatic activity of the brain and body. Recent studies suggest that metabolomics has the potential for diagnostic and prognostic characterization, monitoring, and identifying surrogate markers of a broad spectrum of TBIs (Posti et al. 2017). |
| Uric acid (UA) | A recent study indicates a strong correlation between UA levels and patient outcome, either in hospital or at six months after discharge (Hatefi et al. 2016). |
| Brain derived neurotrophic factor (BDNF) | Assessment of BDNF levels within 24 hr of head injury can predict the severity and prognosis of TBI. Healthy people averaged 60 ng/ml of BDNF in their bloodstreams, whereas patients with brain injuries average less than 20 ng/ml, and those with the most severe TBIs average around 4 ng/ml. High levels of BDNF led to partial recovery within six months of injury and low levels correlated with lingering symptoms at six months (Korley et al. 2016). |
Machine Learning and the Promise for Biomarkers in TBI
Table 1 shows a myriad of attempts by researchers to find a single biomarker that can be used to detect TBI. However, none of them have shown results definitive enough to warrant use in a clinical setting.
Advances in “-omic” profiling technologies allow systematic analysis and characterization of alterations in genes, RNA, proteins, and metabolites. These technologies also offer the possibility of discovering novel biomarkers and pathways activated in disease or associated with disease conditions.
Metabolomic profiling is one such approach that may hold promise in TBI diagnosis, prognosis, and screening. In a study out of Western University in London, Canada, plasma was isolated from 12 concussed and 17 nonconcussed athletes and assayed for 174 metabolites (Daley et al. 2016). Data were analyzed using multivariate statistical analysis and machine learning. Applying machine learning resulted in reducing the number of metabolites required to achieve 92% diagnostic accuracy from 174 to as few as 17. Just as diagnostics for cancer in recent years have leveraged the power of computers to make inferences among enormous sets of clinical data and genomic markers, the future of TBI detection may be solved in a similar fashion.
Motion as a Biomarker
A biomarker is typically thought of as an analyte derived from serum, CSF, saliva, tissue, or other biological substrate that can be sampled. By definition, biomarkers are “objectively measured indicators of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.” They are “intended to substitute for a clinical endpoint (predict benefit or harm) based on epidemiological, therapeutic, pathophysiological, or other scientific evidence” (Biomarkers Definitions Working Group 2001).
Therefore, could motion of the body be such an indicator? With TBI, in fact, it is. Motion itself is the primary cause of TBI, inducing the mechanical insult that damages structures within the brain. That motion, which occurs on impact, can be measured and an alert can be generated when the motion increases above a certain threshold. Several companies have designed products, primarily for athletes, which contain sensitive accelerometers that attach to helmets to function in this way. Some of these companies are Jolt Sensor, X2 Biosystems, and the Shock Box.
Jan Medical has built a device called the BrainPulse, which uses highly sensitive accelerometers placed on a headset to detect movement of the skull from blood flow and pulse in the head. The movement patterns are not only a result of the pulsatile blood flow into the brain but also physical parameters of the brain tissue. Applying machine learning to the data measured by skull motion and disruptions of such motion allow patterns to be recognized and algorithms to be developed that are specific to brain pathologies, including TBI. The BrainPulse data, which are acquired within a few minutes, can aid in the diagnosis of these pathologies and enable triage of patients for adequate treatment.
Treating TBI
Despite improved supportive and rehabilitative care of TBI patients, no effective drugs have been approved for reducing TBI mortality and improving functional recovery. This is because all phase II/III TBI clinical trials so far have failed. Recent preclinical data indicate the potential for restorative therapies targeting multiple cells to stimulate angiogenesis, neurogenesis, axonal sprouting, and oligodendrogenesis, with the goal of improving neurological function after TBI (Xiong et al. 2015). There is also a compelling need to develop novel therapeutics specifically designed to stimulate neuroplasticity, which subsequently promotes neurological recovery after TBI.
Conclusion
TBI is an epidemic with significant health and socioeconomic costs. Despite decades of intense research, little progress has been made in diagnostic, prognostic, and treatment modalities for this indication. This reveals the extreme complexity of the central nervous system and how it responds to injury. Improvements in technology through machine learning and novel biomarkers show promise in the search for valid, portable, and low-cost diagnostic tools, which will hopefully be on the sidelines and at accident scenes in the foreseeable future.
References
Adrian H et al. (2016). Biomarkers of traumatic brain injury: Temporal changes in body fluids. eNeuro 3, ENEURO.0294-16.2016
Bey T and Ostick B (2009). Second Impact Syndrome. West J Emerg Med 10, 1–6.
Biomarkers Definitions Working Group (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69, 89–95.
Daley M et al. (2016). Metabolomics profiling of concussion in adolescent male hockey players: a novel diagnostic method. Metabolomics 12, 185.
Di Pietro V et al. (2017). MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J Neurotrauma 34, 1,948–1,956.
Ezell L. (2013). Timeline: The NFL’s Concussion Crisis. pbs.org/wgbh/pages/frontline/sports/league-of-denial/timeline-the-nfls-concussion-crisis/, accessed July 17, 2018.
Feuerstein G et al. (1994). Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev 6, 341–360.
Hatefi M et al. (2016). Association of serum uric acid level with the severity of brain injury and patient’s outcome in severe traumatic brain injury. J Clin Diagn Res 10, OC20–OC24.
Korley FK et al. (2016). Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J Neurotrauma 33, 215–225.
Kulbe JR and Geddes JW (2016). Current status of fluid biomarkers in mild traumatic brain injury. Exp Neurol 275, 334–352.
Lei J et al. (2015). Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit Care 19, 362.
McInnes K et al. (2017). Mild traumatic brain injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One 12, e0174847.
McKee AC et al. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136 (Pt 1) 43–64.
Neselius S et al. (2012). CSF-biomarkers in Olympic boxing: Diagnosis and effects of repetitive head trauma. PLoS One 7, e33606.
Omalu BI et al. (2005). Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128–134.
Omalu BI et al. (2006). Chronic traumatic encephalopathy in a National Football League player: part II. Neurosurgery 59, 1,086–1,092.
Omalu BI et al. (2017). Postmortem autopsy-confirmation of antemortem [F-18]FDDNP-PET scans in a football player with chronic traumatic encephalopathy. Neurosurgery 82, 237–246.
Papa L et al. (2015). Exploring serum biomarkers for mild traumatic brain injury. In Brain neurotrauma: Molecular, neuropsychological, and rehabilitation aspects, FH. Kobeissy, ed. (Boca Raton, Florida: CRC Press/Taylor & Francis), chapter 22.
Pawlowski A (2017). ‘Concussion’ doctor says kids shouldn’t play these sports until they’re 18. today.com/health/concussion-doctor-warns-against-contact-sports-kids-t115938, accessed July 17, 2018.
Plummer S et al. (2016). The neuroprotective properties of the amyloid precursor protein following traumatic brain injury. Aging Dis 7, 163–179.
Posti JP et al. (2017). Metabolomics profiling as a diagnostic tool in severe traumatic brain injury. Front Neurol 8, 398.
Schallmo MS et al. (2017). AOSSM 2017 Annual Meeting. Assessing trends in the epidemiology of concussions among high school athletes. Orthop J Sports Med 5, suppl 6.
Seifert K (2017). Dr. Bennet Omalu: CTE obsession obscuring truth about brain health of football players. espn.com/nfl/story/_/id/20245394/dr-bennet-omalu-says-obsession-cte-obscuring-larger-truth-brain-health-football-players), accessed July 17, 2018.
Su E et al. (2012). Increased CSF concentrations of myelin basic protein after TBI in infants and children: Absence of significant effect of therapeutic hypothermia. Neurocrit Care 17, 10.1007/s12028-012-9767-0.
Thelin EP et al. (2017). A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir (Wien) 159, 209–225.
Xiong Y et al. (2015). Investigational agents for the treatment of traumatic brain injury. Expert Opin Investig Drugs 24, 743–760.
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions. All trademarks used herein are the property of their respective owner.

