Finding ways to slow the aging process and find the proverbial “fountain of youth” has been a holy grail for old-world explorers and modern-day scientists alike. Ponce de Léon thought he’d found it when he arrived at Florida in 1513. However, he eventually gave up and scientists have picked up the search.
The elusiveness of this goal stems from the intricate genetic and environmental factors that contribute to aging. To better understand the integration of these processes, some researchers have begun to transition from identifying single-gene mutations that contribute to aging to an integrative analysis of the process. These experts propose that systemic factors likely play a major role in the aging of multiple tissues and may be the key to a fountain of youth.
Growth differentiation factor 11 (GDF11) has been investigated as a systemic component of the blood that plays a central role in the aging process. Initial studies showed that GDF11 is lower in old mice and increasing levels of GDF11 regenerated skeletal muscle, brain, and heart tissue, thereby identifying a potential therapeutic target to not only slow but possibly reverse some aging processes and achieve at least prolonged, if not eternal, youth (Sinha et al. 2014, Katsimpardi et al. 2014, Loffredo et al. 2013). However, a study published in the July 2015 issue of Cell Metabolism (Egerman et al. 2015) showed that GDF11 may actually inhibit muscle regeneration, emphasizing that the role of GDF11 in aging — much like the aging process itself — is much more complex than initially thought.
The Study of Aging: A Systemic Approach Beginning in Muscle
Aging is an intricate process involving a multitude of signaling events that promote degeneration at the molecular, cellular, tissue, and organismal levels throughout the body (Wang et al. 2014). Both genetic factors and environmental conditions play a role in age-related decline, which is caused by a combination of stress-induced damage to biological macromolecules, side effects of energy metabolism, deregulation of processes involved in cellular and tissue integrity, and reduced coordination of systemic physiological control (Wang et al. 2014).
Skeletal muscle is frequently studied in relation to aging because it is a highly dynamic tissue, and age-associated loss of skeletal muscle mass (called sarcopenia) is associated with morbidity and mortality from age-related diseases. Furthermore, recent studies (reviewed in Demontis et al. 2013) suggest that skeletal muscle contains growth factors and cytokines that modulate systemic processes, including those in the liver, pancreas, adipose tissue, endothelium, and muscle itself. Furthermore, the prominence of DNA mutations and low levels of antioxidant molecules (which combat oxidative stress associated with metabolism and environmental factors) in skeletal muscle relative to other tissues suggest the earlier onset of age-related degeneration in skeletal muscle may influence the aging of other tissues (Demontis et al. 2013). Therefore, seeking systemic factors that are present in muscle will likely be instrumental to development of a systemic anti-aging therapy.
Heterochronic Parabiosis: A Common Circulatory System to Study Aging
Heterochronic parabiosis, a surgical technique that merges the circulation systems of two animals of different ages by making facing incisions in their respective flanks, has been used in scientific studies for over 150 years. In 1864, French zoologist Paul Bert was the first to describe heterochronic parabiosis when he attached two animals through their skin (for a summary of his experiments, see Conboy et al. 2013). As the wounds healed following the surgical procedure, the capillaries from one animal infiltrated the tissue of the other and established a common circulatory system between the mice.
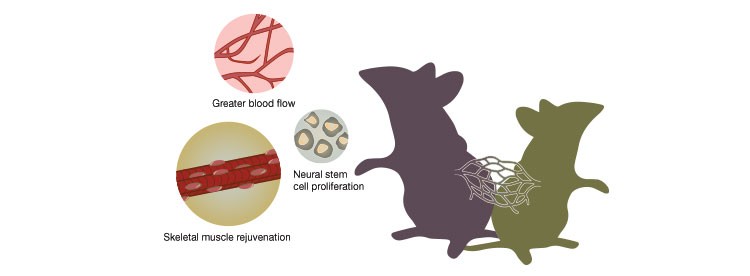
Recent experiments that used heterochronic parabiosis to merge the circulatory systems of an old (left) and young (right) mouse showed that a blood “factor” from the young mouse increased blood flow, neural stem cell proliferation, and skeletal muscle rejuvenation in the old mouse.
In 1950, Cornell University researcher Clive McCay performed heterochronic parabiosis to connect a young mouse to an old mouse. According to McCay’s observations, the old mouse “seemed rejuvenated” following the procedure, although the lack of high-power molecular analysis techniques at that time prevented him from finding a molecular explanation for his observation. Scientists at the time did not pursue this further and over the next 55 years, heterochronic parabiosis was largely ignored as a technique for the study of aging.
In 2005, researchers at Stanford University resurrected parabiosis and showed that merging the circulatory systems of a young and an old mouse caused the skeletal muscle satellite cells and the hepatic progenitor cells of the old mouse to proliferate and regenerate much like those of a young mouse (Conboy et al. 2005). Specifically, the old mice had increased activity of the Notch signaling pathway, which is involved in activation of skeletal muscle stem cells and decreases with age, as well as restored formation of the cEBP-α complex, which promotes regeneration of hepatic progenitor cells. However, the precise systemic factors in the blood that contributed to the results, that is whether the observed effects were due to an upregulation of regeneration factors or a downregulation of degradation factors, was still unclear.
GDF11: The Anti-Aging Factor in the Blood
As research continued to investigate possible factors responsible for the aging process, GDF11 began to emerge as a key player in the aging process. Using heterochronic parabiosis, Loffredo and colleagues showed exposure to the young mouse’s serum drastically reduced cardiac hypertrophy, cardiomyocyte size, and molecular remodeling in the paired old mouse (Loffredo et al. 2013). Further investigation of the serum using slow off-rate modified aptamer-based proteomics showed that GDF11 was a circulating factor that was relatively abundant in young mice and significantly depleted in old mice. The authors also showed that intraretroperitoneal injection of recombinant GDF11 reversed age-related cardiac hypertrophy in mice in vivo, further indicating that GDF11 had a direct anti-hypertrophic effect on cardiomyocytes.
A similar investigation in skeletal muscle showed that increasing GDF11 by either heterochronic parabiosis or systemic injection restored age-related functional impairments and genomic integrity in the skeletal muscle satellite cells of the old mice (Sinha et al. 2014). The GDF11 also increased muscle structure and function and improved strength and endurance exercise capacity in aged mice. Another study by Katsimpardi and colleagues showed that parabiotic exposure to young serum was associated with increased blood flow, activation of the subventricular zone of the brain, neural stem cell proliferation, and olfactory neurogenesis in older mice. In addition, exposure to GDF11 in vivo increased the volume of blood vessels in the brain and the proliferation of primary brain capillary endothelial cells in vitro (Katsimpardi et al. 2014). Taken together, these studies indicated that GDF11 is a systemic factor with an anti-aging effect on multiple key tissues throughout the body.
GDF11: A Degenerative Molecule?
Although the studies cited above strongly suggested GDF11 as a novel multitargeted anti-aging molecule, much of the research on the role of GDF11 in aging tissues was limited to a few laboratories, most of them at Harvard Medical School and the Harvard Stem Cell Institute. To confirm the reproducibility of the results, Egerman and colleagues at the Novartis Institutes for Biomedical Research in Cambridge, MA attempted to repeat the experiments performed by the Harvard scientists. However, they reported an inability to reproduce the results from the Harvard group and indicated that GDF11 may actually promote aging, as serum GDF11 tended to be higher, not lower, in older mice (Egerman et al. 2015). Furthermore, systemic administration of GDF11 did not improve muscle regeneration or satellite cell function in older mice and delayed muscle regeneration in younger mice due to reduced expansion and differentiation of the cells.
Additionally, the Novartis group found that the SOMAmer analysis used by the Harvard researchers was not specific to GDF11, as it was unable to differentiate between GDF11 and myostatin, a closely related protein. Because GDF11 and myostatin use the same receptor and canonical signaling pathway, the Novartis group also investigated whether different concentrations of each protein would have distinct effects on cell signaling and function. They showed that both proteins equivalently activated the canonical SMAD2/3 pathway and the non-canonical MAPK pathways to inhibit muscle growth and myoblast differentiation. Microarray analysis also showed no difference between myoblasts treated with GDF11 and myostatin. From these results, the authors concluded that GDF11 reduces skeletal muscle satellite cell function and is virtually indistinguishable from myostatin, a well-known inhibitor of skeletal muscle proliferation and regeneration (McPherron et al. 1997). Furthermore, the authors propose GDF11 as a target for pharmacologic blockade, rather than upregulation, to slow the aging process.
Reasons for the GDF11 Controversy
Such conflicting results have questioned the status of GDF11 as an anti-aging molecule and how GDF11 should be manipulated to slow the aging process. GDF11 is a member of the transforming growth factor beta (TGF-β) superfamily of proteins that control functions such as proliferation and differentiation in a variety of cell types. The mature proteins of GDF11 and myostatin exhibit 89% identity in their amino acid sequences, which had caused some scientists to question the regenerative capacity of GDF11 even before publication of the data from the Novartis group. The Novartis group administered GDF11 to young mice and found that muscle regeneration was impaired following muscle damage with snake venom toxin, suggesting that, much like its close relative myostatin, GDF11 has an inhibitory effect on muscle growth.
The Antibody or the Dose?
Amy Wagers, who led much of the work at Harvard University, noted in a May 2015 article in Science that she and her colleagues used a different antibody to distinguish GDF11 from myostatin and that the Novartis researchers used a dose of GDF11 that was three times higher than that used by Wagers and her colleagues (Kaiser 2015). According to Wagers, the signaling pathway that GDF11 is involved in “is notoriously dose-sensitive,” with low and high doses possibly producing opposing effects. Furthermore, she indicated that the two studies are not comparable because the snake venom toxin used to induce skeletal muscle injury may have been more likely to damage muscle stem cells than the Harvard team’s method of freezing the tissue.
While researchers outside of the Harvard and Novartis groups hesitate to support the notion of GDF11 as a pro-aging molecule based on a single investigation, they do suggest that the new study may in part challenge the original work on GDF11 by the Harvard scientists. Also, the Novartis group did not investigate the effects of GDF11 on other tissues, such as those in the cardiovascular and central nervous systems. Harvard neuroscientist Lee Rubin, who led the work showing that GDF11 increased neural stem cell proliferation and volume of blood vessels in the old mouse brain, reported to Science magazine that he and his colleagues are designing experiments to “convince ourselves that what we see in the brain is real” (Kaiser 2015).
Conclusion
Both the Novartis and Harvard groups, as well as research experts outside of those two institutions, agree that blood from young mice rejuvenates old mice. Whether GDF11 is the rejuvenating factor is controversial, and many experts in muscle regeneration agree that the study by the Novartis group has challenged the notion of GDF11 as the central “anti-aging” molecule. However, the physiological process of aging is complex, and according to Se-Jin Lee, a molecular biologist at Johns Hopkins University who studies myostatin, the effects of GDF11 in the body are likely complex and the findings from the Novartis group may not completely contradict the conclusions of the Harvard group. Lee indicated that before GDF11 is considered as a therapeutic target for reversing the aging process, further research is needed to understand how GDF11 works systemically. These studies will likely include clarifying the effects of concentration on cellular function and the possible existence of different tissue-specific isoforms.
References
Conboy IM et al. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433,760–764.
Conboy MJ et al. (2013). Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 12, 525–530.
Demontis F et al. (2013). The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12, 943–949.
Egerman MA et al. (2015). GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab 22,164–174.
Kaiser J (2015). ‘Rejuvenating’ protein doubted. Science 348, 6237.
Katsimpardi L et al. (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634.
Loffredo FS et al. (2013). Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839.
McPherron AC et al. (1997). Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90.
Sinha M et al. (2014). Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652.
Wang L et al. (2014). Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol 217, 109–118.
SOMAmer is a trademark of SomaLogic, Inc.

