Recent advances in chimeric antigen receptor (CAR) T-cell therapy have opened exciting new avenues for the treatment of a variety of malignancies. Cytokine release syndrome (CRS), a powerful immune response to activated CAR T cells, is the primary side effect of CAR T-cell therapy, but existing CRS treatments can make CAR T-cell treatment less effective. In two recent studies, researchers used multiplexed cytokine immunoassays to better understand how CAR T-cell therapy triggers CRS and to identify several promising strategies for early intervention in CRS development.
Cytokine Release Syndrome: The Dark Side of CAR T-Cell Therapy
CAR T-cell therapy offers incredible promise for a number of challenging fields in medicine. While it can be used to treat infectious diseases, autoimmune disorders, cardiometabolic disease, senescence, and others (Aghajanian et al. 2022), CAR T-cell therapy has been widely applied in the field of oncology, with several therapies already approved by the U.S. Food and Drug Administration (FDA) for treating a variety of cancers (Cappell and Kochenderfer 2023).
Because CAR T cells are engineered to possess enhanced properties like highly specific detection, robust signaling, or high proliferation potential, they can easily disrupt effective immune responses. Typically, activated T cells attack the offending agent with cytotoxic chemicals while also secreting signaling molecules to recruit bystander immune and endothelial cells, which send out their own chemical signals that increase the toxicity of the response and recruit additional responders. Once the threat is neutralized, regulating cells and signals blunt the immune response before collateral damage is done to the surrounding cells.
CAR T cells can upset this balance by inducing a powerful immune response — cytokine release syndrome (CRS; also known as cytokine storm) — that overwhelms negative feedback mechanisms and results in significant toxicities (Fajgenbaum and June 2020) (Figure 1). CRS is the primary side effect of CAR T-cell therapy. Recent clinical trials report that most patients are likely to experience some amount of CRS, with as many as one in four suffering from severe symptoms that can also be fatal (Jin et al. 2018).
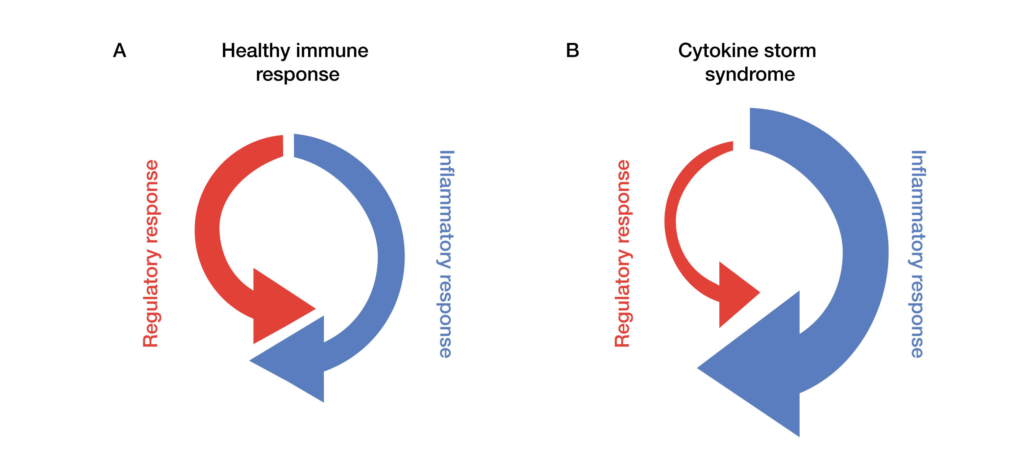
Fig. 1. Regulatory and inflammatory responses in healthy immune systems and those affected by cytokine storm syndrome. In a standard immune response (A), a proinflammatory chemical cascade activates the immune system to initiate and process cell death. This is balanced by regulatory and mediating chemical signals that keep the response localized and manageable. In contrast, cytokine storms (B) are characterized by overly robust inflammatory responses, weakened regulatory action, or both. CAR T-cell therapies, engineered for their strong inflammatory response, are particularly susceptible to cytokine storms.
The Challenge of Treating CRS in Oncology
Early clinical trials testing CRS treatments in oncology were informed by CRS treatments in other contexts, such as septic shock, macrophage activation syndrome, and infectious diseases (Borrega et al. 2019). However, the unique pathophysiology of CAR T cell–related CRS presents unique complications for existing treatment protocols (Fajgenbaum and June 2020). Corticosteroids used to treat severe CRS by calming the immune system can also blunt the therapeutic effect of the CAR T cells and leave patients susceptible to post-treatment infections (Shubert et al. 2020). The more conservative blockade of IL-6 signaling with tocilizumab can only be used in advanced CRS, and because tocilizumab does not cross the blood-brain barrier, it is ineffective at treating or preventing cytokine-related neurotoxicity and may even exacerbate it (Borrega et al. 2019; Shubert et al. 2020). A deeper understanding of the pathophysiology of CAR T cell–related CRS is needed to develop better strategies for managing this serious side effect without compromising the therapeutic effect of the therapies themselves.
Measuring Cytokines to Characterize CRS and Identify Therapeutic Targets
Many cytokines are secreted amidst CRS, including IFNγ, TNAα, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-12 (David et al. 2021). Therefore, analyzing these cytokines at the proteome level can provide a deeper view into how cytokine networks marshal the immune system as they react and respond in real time during CRS. Multiplex immunoassays, which can measure multiple cytokines simultaneously, are uniquely positioned to provide useful insights for understanding how CAR T-cell treatments trigger the cytokine storm (David et al. 2021). Reproducible, rapid, and sensitive multiplexed immunoassays with wide dynamic ranges have been developed to enable researchers to take a systems-level view of CRS progression.
In a recently published study, Leclercq-Cohen et al. (2023) profiled the cellular and molecular players involved in CRS and analyzed the impact of several different cytokine blockade strategies in whole blood and in an in vivo diffuse large B-cell lymphoma model in immunocompetent humanized mice. After treatment with T cell–engaging therapy, the researchers studied the timing of cytokine release using the Bio-Plex Pro Human Chemokine Panel from Bio-Rad. Flow cytometry and scRNA-Seq of whole blood and bulk RNA-Seq of endothelial cells exposed to induced cytokine release were further leveraged to characterize how the signaling cascades impacted immune cell presence and proliferation. These assays revealed increases in IFNγ, TNFα, IL-2, IP-10, MCP-1, MIP-1α, MIP-1β, IL-1β, and IL1Ra as early as 6 hr post-treatment in whole blood samples, with IL-6 and IL-8 peaking after 20 hr.
The effects of several different cytokine-blocking strategies targeting some of these early-rising cytokines were then tested in whole blood and in the animal model. The steroid dexamethasone, the TNFα blocker adalimumab, an NLRP3 inhibitor, and an IL-1R inhibitor all effectively lowered the downstream levels of both T cell–derived and myeloid-derived cytokines and all, with the exception of adalimumab, did not interfere with the anti-tumor efficacy of the T cell–engaging therapy. Altogether, this work, enabled by multiplexed cytokine immunoassays, was able to identify several promising cytokines to target for early intervention in CRS development.
Improving CAR T-Cell Safety by Altering Cytokine Profiles
Another strategy for improving both the efficacy and the safety profile of CAR T-cell therapies is to alter the cytokine profile of the initial T-cell response itself by including transgenic cytokine release cassettes into the engineered T cells. This approach allows scientists to shape the immune response from the earliest moments after T-cell activation. Chmielewski and Abken (2017) deployed this strategy on a CEA+ pancreatic tumor model system in immunocompetent mice. Through systematic screening of several pro-inflammatory cytokines, they determined that IL-18 and IL-21 produced signatures favoring acute pro-inflammatory responsiveness and resistance to exhaustion, with IL-18 demonstrating the most promising profile. Conversely, other cytokines including TGF-β1, IL-12, and IL-10 produced mixed or poor signatures.
Follow-up experiments involved treating mice with several different anti-CEA CAR T cells to assess the impact of CAR T cells engineered with an inducible IL-18 (iIL-18) expression cassette on survival. Compared to inducible IL-12 (iIL-12), mixed (iIL-18 + iIL-12), and non-expressing CAR T cells, iIL-18 CAR T cells were associated with prolonged survival. Analysis of the cytokine profile with a Bio-Rad Bio-Plex Pro mouse 17-plex immunoassay revealed that iIL-18 CAR T-cell treatment was accompanied by an increase in serum IL-6, IL-22, and IL-27 levels, while serum IL-18 levels increased only slightly, excluding a major systemic IL-18 release. Additionally, no change was observed in serum IFN-γ levels.
This cytokine pattern was indicative of a stronger acute inflammatory response than the profiles seen in the controls, an outcome that was confirmed by increased infiltration of activated dendritic cells and natural killer cells, and fewer T regulatory cells and M2 macrophages in tumor samples collected days after administration of T-cell therapy. By shaping the cytokine milieu from the first moment of activation, the researchers achieved a stronger and more durable local reaction coupled with a smaller systemic reaction — a reaction profile likely to be protective against systemic side effects like CRS in clinical applications.
Advancing CRS Therapeutics Discovery and Development with Multiplexed Immunoassays
While the widespread adoption of CAR T-cell therapies is limited in part by the severity of possible adverse effects such as CRS, the studies highlighted here demonstrate that significant progress is underway in the development of novel, improved treatment strategies for this serious side effect. The work, centered around understanding the core processes at work when engineered T cells interact with the native immune system, represents an important step toward identifying early cytokine storm biomarkers and developing mitigation strategies, allowing gentle interventions that preserve the therapeutic effect of T-cell treatments.
Crucial to each study was the use of multiplexed cytokine immunoassays, which enabled a thorough assessment of the complete cytokine profile associated with CRS in each of the utilized research models. Indeed, analyzing cytokines together is the only way to fully understand immune-related responses and identify cytokine biomarkers because these molecules function together in networks to regulate immune responses. These assays are a critical tool in the CRS research toolbox and will help address historical challenges associated with CAR T-cell therapy.
Learn more about Bio-Plex Multiplex Immunoassays.
References
Aghajanian H et al. (2022).CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat Metab 4, 163–169
Borrega JG et al. (2019). In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. Hemasphere 3, e191.
Cappell KM and Kochenderfer JN (2023). Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol 20, 359–371.
Chmielewski M and Abken H (2017). CAR T cells releasing IL-18 convert to T-bethigh FoxO1low effectors that exhibit augmented activity against solid tumors. Cell Rep 21, 3205–3219.
David P et al. (2021). An overview of proteomic methods for the study of ‘cytokine storms’. Expert Rev Proteomics 18, 83–91.
Fajgenbaum DC and June CH (2020). Cytokine storm. N Engl J Med 383, 2255–2273.
Jin Z et al. (2018). The severe cytokine release syndrome in phase I trials of CD19-CAR-T cell therapy: a systematic review. Ann Hematology 97, 1327–1335.
Leclercq-Cohen G et al. (2023). Dissecting the mechanisms underlying the cytokine release syndrome (CRS) mediated by T-cell bispecific antibodies. Clin Cancer Res 29, 4449–4463.
Shubert M-L et al. (2020). Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol 32, 34–48.