
Navigating Change: Public Health Labs Are Adapting to New Regulations for Laboratory-Developed Tests
Labs face a growing compliance burden and increased uncertainty due to new regulations surrounding laboratory-developed tests (LDTs). The U.S. FDA’s final rule and Supreme Court’s recent decision to overturn the Chevron doctrine are creating a complex regulatory environment that challenges the ability of public health labs to rapidly respond to emerging pathogens. This article explores the implications of these changes and more.

Compliance and Regulations for Flow Cytometry in Drug Discovery: Webinar Series
In this webinar series, learn about 21 CFR Part 11 regulations for flow cytometry in drug discovery. Learn what software and instruments can help you comply.
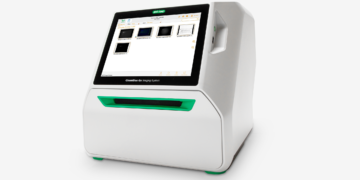
Compliance at Your Fingertips: Using Image Lab Touch Software for Digital Imaging in Regulated Environments
Maintaining compliance while leveraging advanced digital imaging technologies can be a daunting task. Luckily, Bio-Rad Image Lab Touch Software takes this challenge head-on by offering a seamless solution for digital imaging. Learn how Image Lab Touch Software integrates with Bio-Rad imaging systems to support researchers in meeting stringent regulatory requirements, ensuring data integrity and security without compromising efficiency or ease of use.

Cytometry in Drug Discovery: An Introduction to Regulation, Standardization, and Productivity
Many researchers learn flow cytometry in academia, but find new challenges in the commercial sector. In this webinar, learn about regulatory issues and more.
