Western blotting, the backbone of protein research, is a universal lab procedure. While the premise of a western blot is simple — target proteins are identified and quantitated via antibody-antigen interactions — the traditional workflow is labor intensive and time consuming. Researchers have long sought a faster solution — an archetype that would streamline the entire process of separation, transfer, and visualization of results without compromising data quality.
The recently launched V3 Western Workflow, consisting of TGX Stain-Free™ precast gels, the Trans-Blot® Turbo™ transfer system, and the ChemiDoc™ MP imager, is a ground-breaking solution to traditional western blotting benchwork. It eliminates inherent western blotting uncertainty by providing confirmation of results at three key checkpoints: protein separation, transfer to the membrane, and western blot quantification. The proprietary in-gel chemistry greatly speeds the procedural workflow by eliminating the need for Coomassie and Ponceau S staining (see Figures 1 and 2) while offering an alternative to housekeeping protein normalization. The combination of the gel chemistry with stain-free technology — along with each component of the system — greatly streamlines the workflow.
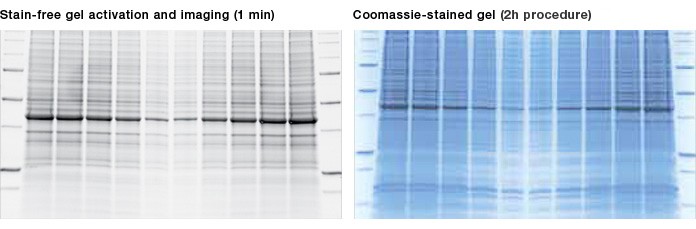
Fig. 1. Results using a stain-free gel are similar to those using a Coomassie-stained gel. Criterion™ TGX Any kD Stain-Free™ precast gels run at 200 V for 45 min. Stain-free technology is visualized on the ChemiDoc MP imaging system and compared with a gel stained overnight with Bio-Safe™ Coomassie stain.
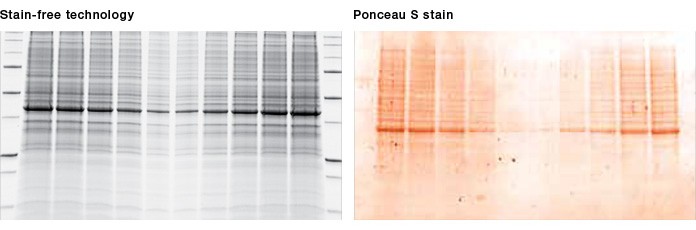
Fig. 2. Visualization of proteins after transfer. Once stain-free technology is activated during gel imaging, proteins can be visualized on the membrane, allowing instant verification of protein transfer. Ponceau S staining of the membrane is no longer required.
Many protein researchers, like Dominic Mancini, a technical research assistant and lab management specialist in the Channing Laboratory at Harvard Medical School, share the frustrations of traditional western blotting. Mancini uses protein expression studies to define patterns that can distinguish between normal and diseased states. Here, he explores the utility of the V3 Western Workflow as it pertains to the Channing Lab’s research questions concerning chronic obstructive pulmonary disease (COPD).

Dominic Mancini, technical research assistant and lab management specialist at Harvard Medical School, in front of the ChemiDoc MP imaging system. The system allows him to visualize the gel and blot during different stages of western blotting in record time.
COPD is a progressive disease strongly influenced by cigarette smoking and genetic predisposition. COPD is the third leading cause of death in this country (Croxton et al. 2002). Despite aggressive smoking prevention and cessation efforts, one-quarter of Americans continue to smoke (Zhou et al. 2012). The goal of the Channing Lab’s research is to better understand COPD genetic determinants by exploring how certain candidate genes interact with environmental factors. Genes were selected based on the results of the COPD genome-wide association study (GWAS).
Visualize. Verify. Validate.
“I deal primarily with mouse research and it takes a while to generate the animals for each experiment, and even longer to carry out the experiment,” says Mancini. “The V3 system saves me a load of time and that equates to money.”
The Channing Lab investigates a variety of proteins expressed in human lung epithelial cells, including hedgehog interacting protein (HHIP) (78 kD) and FAM13A (116 kD). Cell lines are first transfected to overexpress or silence these genes and then treated with cigarette smoke. Various pathways are subsequently studied to determine the effects on upstream regulators and downstream targets. (Hedgehog, for instance, is a crucial signaling pathway for the development of the lungs.) In vivo validation is performed in mouse models, and ex vivo via the primary human cells obtained from biopsies or bronchial brushings.
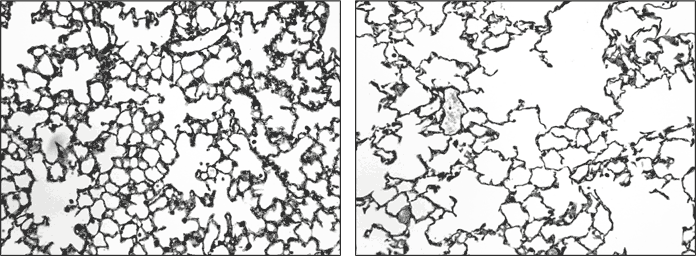
Fig. 3. Healthy and diseased lung tissues. Images of healthy (left) and COPD (right) lung tissues, magnified 200x, and treated with Gill’s stain. Macrophages are more pronounced in COPD tissue. Images courtesy of the Channing lab.
Mancini explains that before the V3 Western Workflow, he would pour his own gels, stain gels with Coomassie blue, perform an overnight tank transfer onto a PVDF membrane, do all the primary and secondary antibody incubations and washes, and finally develop the blot using a substrate. Typically the total workflow would take between two to three days. Today the workflow takes half the time using the V3 Western Workflow. He further notes that by using the Trans-Blot Turbo transfer system he can complete a western blot within a day.
“We do a lot of fishing expeditions in the lab,” says Mancini. “Sometimes we’re stripping a blot and reprobing it for proteins in a wide range of sizes. The data I have collected using stain-free technology make me more than a believer.”
Stain-Free Offers an Alternative to Housekeeping Protein Normalization
Traditionally, western blot normalization is performed by probing the membrane with antibodies against a housekeeping protein (HKP) such as β-actin; this process requires stripping the membrane prior to reprobing with the HKP antibodies, or performing fluorescense-based multiplex immunodetections. (The Channing Lab currently uses only chemiluminescence detection.) Stain-free technology allows for normalization by directly measuring the total amount of protein bound to the membrane (Short R et al. 2011). (The technology is based on the proprietary chemistry found in the TGX Stain-Free precast gels.) In addition, for the Channing Lab, the stain-free technology provided a workaround solution for low actin COPD samples, as well as other samples where β-actin won’t work for normalization.
“The data for the β-actin samples correlate nicely with the stain-free samples,” says Mancini. “Both methods agree with each other, and where we can’t use one method, the other is a great alternative. Going stain-free has the benefit of being a great time saver.”
Since its inception in 1979, the western blot has become an inextricable process in the exploration and confirmation of proteins. Improved technology facilitates higher throughput, potentially leading to a clearer understanding of molecular events and elucidation of cellular pathways critical in COPD pathogenesis. As demonstrated by the Channing Lab, the V3 Western Workflow is a revolutionary solution that streamlines procedures while ensuring confidence.
References
Croxton TL et al. (2002). Future Research Directions in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 165, 838 -844.
Short R et al. (2011). Stain-free approach for western blotting: alternative to the standard blot normalization process. Bio-Rad Bulletin RP0051.
Zhou X et al. (2012). Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet 21, 1325-35.
Coomassie is a trademark of BASF Aktiengesellschaft.
SYPRO is a trademark of the Invitrogen corporation.

