Introduction
Protein quantitation is an important part of many workflows. The estimation or quantitation of the protein in a sample frequently allows researchers to understand the results from their work and make decisions on the subsequent steps. Bio-Rad offers four colorimetric assays for protein quantitation: the Quick Start™ Bradford protein assay, the Bio-Rad protein assay, the DC™ protein assay, and the RC DC™ protein assay. Knowing which one is the appropriate kit to use and what to watch out for can save time and prevent frustration. Here are the answers to some of the most frequently asked questions related to the use of these kits.I want to quantify samples in my protein extraction buffer. Which kit should I select?
All Bio-Rad protein assay kits can be used to quantitate samples in common buffers and salts. But some of the kits, such as the Quick Start™ Bradford protein assay or the Bio-Rad protein assay, are sensitive to many detergents. The DC protein assay can be used to quantitate proteins in the presence of various concentrations of detergents in addition to many common reagents. The RC DC protein assay is both reducing agent compatible (RC) and detergent compatible (DC). To see which kit is compatible with specific contaminating compounds, see Bulletin 1069 or the product manual at www.bio-rad.com. If the protein is in the presence of a compound that has not been tested and this compound can not be removed before quantitation, it would be best to prepare a standard protein dilution series in a similar buffer to determine the effect of the buffer composition on the assay (see Bulletin 1069).Bio-Rad offers kits with BSA and γ-globulin as control proteins. How do I select a protein standard?
In any protein assay, the best protein to use as a standard is a purified preparation of the protein being assayed. In the absence of such an absolute reference protein, another protein must be selected as a relative standard. The best relative standard to use is the one that gives a color yield similar to that of the protein being assayed. Selecting such a protein standard is generally done empirically. Alternatively, if only relative protein values are desired, any purified protein may be selected as a standard. If a direct comparison of two different protein assays is being performed, the same standard should be used for both procedures. Bio-Rad offers two standards: bovine γ-globulin (standard I) and BSA (standard II). With the Quick Start Bradford and Bio-Rad protein assays, the dye color development is significantly greater with albumin compared to most other proteins, including γ-globulin (Figure 1). Therefore, the BSA standard would be appropriate if the sample contains primarily albumin, or if the protein being assayed gives a similar response to the dye. For a color response that is typical of many proteins, the γ-globulin standard is appropriate. The DC and RC DC protein assays show little difference in color development between BSA and γ-globulin. It is recommended, however, that the same standard be used if comparisons are to be made between different assays.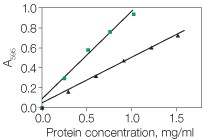
Fig. 1. Typical standard curves for the Bio-Rad protein assay: bovine serum albumin (![]() ) and γ-globulin (
) and γ-globulin ( ![]() ).
).

