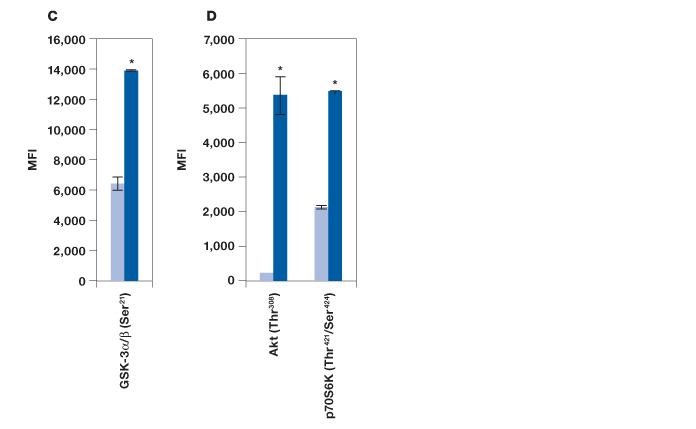Abstract
Using Luminex xMAP technology we have developed new magnetic bead–based immunoassays, in addition to our current panels of cell signaling (phosphorylated and unphosphorylated), and GAPDH and β-actin housekeeping proteins. Of these assays, two multiplex panels were configured for convenient studies of Akt and MAPK signal transduction pathways. Using these assays, we studied the effects of insulin, EGF (epidermal growth factor), and UV radiation on 21 signal transduction proteins. Insulin treatment resulted in a significant increase in phosphorylation of insulin receptor-β (IR-β), IRS-1, Akt, mTOR, BAD, GSK-3α/β, p70 S6 kinase (p70S6K), and S6 ribosomal protein (S6RP), but not PTEN (Figure 2). EGF treatment led to the phosphorylation of EGF receptor and the orphan receptor HER-2 (Figure 6). Receptor activations were accompanied by phosphorylation of nine downstream effectors; MEK1, Erk1/2, p90 RSK, ATF-2, c-Jun, Stat3, p38 MAPK, JNK, and HSP27 (Figure 5). UV radiation resulted in a markedly different phosphorylation profile; MEK1, Erk1/2, and p90 RSK showed little or no response, while p38 MAPK, JNK, and p53 were strongly phosphorylated (Figure 5). These changes in phosphorylation are in agreement with published literature and confirm the exceptional sensitivity and specificity of the Bio-Plex Pro cell signaling assays in mapping intricate Akt and MAPK pathways. Finally, it should be noted that the assays described in this publication were performed with less than two 96-well plates and can be accomplished in less than 48 hr by one investigator.
Introduction
Protein kinases are among the largest protein families in eukaryotes, with more than 500 identified (Manning et al. 2002). These proteins induce changes in metabolism, transcription, cell cycle progression, cytoskeletal rearrangement, cell movement, apoptosis, and differentiation through phosphorylation of substrate proteins (Chang and Karin 2001). Protein phosphorylation also plays a critical role in intercellular communication during development and in the functioning of the nervous and immune systems (Boulanger et al. 2001 and Yates 2011). Mutations in many of these kinases appear to be associated with human diseases and are thus the subject of intense research.
We have developed new cell signaling assays for the semiquantitative detection of 41 phosphoproteins, 18 total proteins (both phosphorylated and unphosphorylated forms of the target proteins), and two housekeeping proteins, GAPDH and β-actin. A detailed list is available at www.bio-rad.com/cellsignalingassays. These Bio-Plex Pro assays utilize Luminex xMAP technology (Dale et al. 2008), employing a magnetic bead–based workflow to detect multiple proteins in a single well of a 96-well microplate with high sensitivity, reproducibility, and ease.
Of these assays, two multiplex panels were configured for the convenient studies of Akt (also known as protein kinase B or PKB) and mitogen-activated protein kinase (MAPK) pathways (Manning et al. 2007 and Morrison 2012). The Bio-Plex Pro cell signaling Akt panel comprises eight key phosphoproteins located upstream and downstream from Akt and includes: Akt (Ser473), BAD (Ser136), GSK-3α/β (Ser21/Ser9), IRS-1 (Ser636/Ser639), mTOR (Ser2448), p70 S6 kinase (Thr389), PTEN (Ser380), and S6 ribosomal protein (Ser235/Ser236). The Bio-Plex Pro cell signaling MAPK panel is composed of nine proteins phosphorylated in response to mitogens, cytokines, and stress: ATF-2 (Thr71), Erk1/2 (Thr202/Tyr204, Thr185/Tyr187), HSP27 (Ser78), JNK (Thr183/Tyr185), MEK1 (Ser217/Ser221), p38 MAPK (Thr180/Tyr182), p53 (Ser15), p90 RSK (Ser380), and Stat3 (Ser727). Here, we report the activation of signal transduction pathways in cell cultures treated with insulin, EGF, and UV radiation using the two panels.
Materials
The Bio-Plex Pro cell signaling Akt 8-plex and the Bio-Plex Pro cell signaling MAPK 9-plex panels (catalog #LQ0-0006JK0K0RR and #LQ0-0000S6KL81S) are provided as ready-to-use kits. The kits include antibody-coupled magnetic beads, detection antibodies, cell wash buffer, cell lysis buffer, cell lysis factor QG, detection antibody diluent, streptavidin-PE, bead resuspension buffer, wash buffer, a 96-well flat bottom plate, sealing tapes, and a quick guide. The panels are provided with four positive cell lysate controls. The Akt kit includes NIH 3T3 treated with PDGF, HEK-293 treated with EGF, SK-BR-3 treated with EGF, and PC12 treated with NGFβ, while the MAPK kit includes HEK-293 treated with UV, HEK-293 treated with EGF, SK-BR-3 treated with EGF, and HeLa treated with IFN-α. Kits are provided with background cell lysate control (phosphatase-treated HeLa lysate). The following cell lines were used in this study: Hep G2 (derived from hepatocellular carcinoma), HEK-293 (derived from adenovirus-infected embryonic epithelial cells), and SK-BR-3 (derived from breast adenocarcinoma overexpressing the HER-2/c-erbB-2 gene product). All were purchased from American Type Culture Collection (ATCC) (catalog #HB-8065, CRL-1573, and HTB-30). Human recombinant insulin and EGF were purchased from Santa Cruz Biotechnology and EMD Millipore.
Methods
Cell Culture and Treatment
Cell lines were cultured according to ATCC guidance. Hep G2 and SK-BR-3 cells were cultured in serum-free media for 18 hr prior to treatment with 1 μg/ml insulin for 30 min and 75 ng/ml EGF for 7.5 min. HEK-293 cells were exposed to 30 mJ UV using Stratalinker UV 2400 and harvested after 2 hr incubation with growth media. Cells were lysed using reagents and protocols from the Bio-Plex Pro cell signaling kits. Protein concentration was measured and adjusted to 0.2 mg/ml using Bio-Rad’s DC™ protein assay kit (catalog #500-0112). In cases where higher sensitivity is required, testing with higher protein concentration should be tried.
Bio-Plex Multiplex Immunoassays
The principle of the Bio-Plex Pro bead–based assays is similar to capture sandwich immunoassays (Zhou and Geng 2011 and Yeung et al. 2011). The capture antibody–coupled beads are first incubated with the sample followed by incubation with biotinylated detection antibodies. After washing away the unbound biotinylated antibodies, the beads are incubated with a reporter streptavidin-phycoerythrin (SA-PE) conjugate. The beads are then passed through the Bio-Plex® array reader, which measures the fluorescence of the bound SA-PE on each bead. Measurements are provided as the median fluorescence intensity (MFI) for a given bead population. Bead populations are identified by fluorescence to determine the analyte being measured. All assay incubations are performed at room temperature as described in the instruction manual for Bio-Plex Pro cell signaling assays (Bio-Rad part number 10024929). In our study, all washes were performed using a Bio-Plex Pro wash station with cycles of 200 μl of wash buffer per well. Assays were developed using the Bio-Plex 200 system at high photomultiplier tube (PMT) voltage setting. The assays are fully compatible with a low PMT setup and the other Bio-Plex readers: Bio-Plex 3D suspension array system and Bio-Plex® MAGPIX™ array readers. Data acquisition was done using Bio-Plex Manager™ 6.1 software.
Assay Performance Characteristics
All Bio-Plex Pro cell signaling assays, including both panels, exhibited intra- and inter-assay %CVs (percentage coefficients of variation) of <15% and <25%, respectively. Multiplexing compatibility was extensively studied. Assays with cross-reactivity or interference >10% are not recommended for multiplexing. Refer to the Bio-Plex Pro cell signaling assays product information sheet for more details (Bio-Rad bulletin 6285). In addition, the assays were evaluated for specificity, sensitivity, dynamic range, working assay range, and species specificity. A detailed description of methodology was provided previously (Peretz et al. 2013a).
Applications
Elucidating Akt Pathway Activation in Response to Insulin
The PI3K/Akt/mTOR pathway has a critical regulatory role in diverse cellular processes, including cell proliferation, apoptosis, and angiogenesis, and has been implicated in diabetes and cancer (Manning et al. 2007). Figure 1 provides the schematic representation of this pathway.
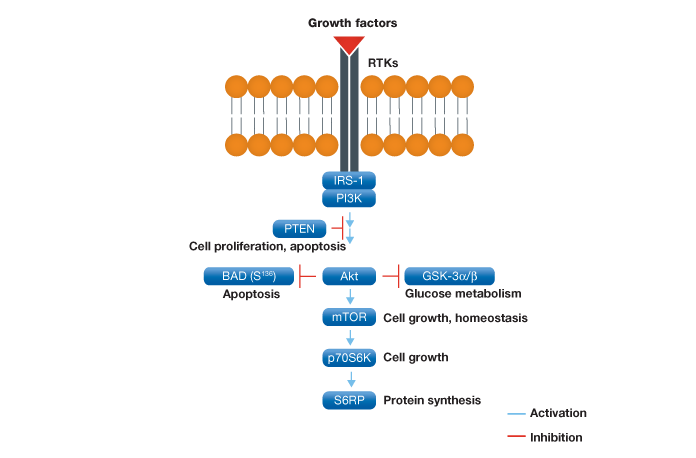
Fig. 1. Schematic representation of the Akt signaling pathway. Following activation at the plasma membrane and subsequent regulation by IRS-1 and PI3K, Akt plays a key role in a variety of cell signaling pathways such as apoptosis, glucose metabolism, and cell growth.
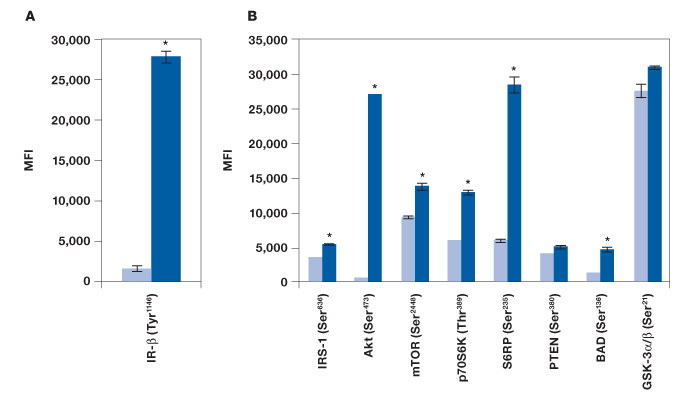
To evaluate the performance of the Bio-Plex Pro cell signaling panel, Hep G2 cells were treated with insulin, a key activator of the Akt pathway, and analyzed with the following assays: IR-β (Tyr1146), Akt 8-plex panel, and a custom 2-plex Akt (Thr308) and p70 S6 kinase (Thr421/Ser424) panel. As expected, a strong phosphorylation response was observed for IR-β (Figure 2, A). A clear, over twofold increase was observed for Akt (Ser473), Akt (Thr308), p70 S6 kinase (Thr389), p70 S6 kinase (Thr421/Ser424), S6 ribosomal protein (Ser235), and BAD (Ser136) (Figure 2, B and D). A significant response was also observed for IRS-1 (Ser636) and mTOR (Ser2448), but not for PTEN (Ser380) and GSK-3α/β (Ser21) (Figure 2, B). Since GSK-3α/β (Ser21) untreated and treated readouts were saturated at ~30,000 MFI, the same plate was reread at low PMT voltage after bead resuspension in 100 μl of resuspension buffer. At low PMT clear discrimination was observed that is consistent with Akt activation of GSK-3α/β (Figure 2, C) (Dummler and Hemmings 2004).
Fig. 2. Detection of protein phosphorylation using the Bio-Plex Pro cell signaling panel on Hep G2 cells treated with insulin. Untreated (■) and insulin-treated (■) cells were harvested to obtain a final protein concentration of 0.2 mg/ml (50 μl/well) by (A) IR-β (Tyr1146) assay, (B) Akt 8-plex panel, (C) reread of B at low PMT, and (D) 2-plex panel that included Akt (Thr308) and p70 S6 kinase (Thr421/Ser424). *P
Measurements of protein phosphorylation may be biased by protein degradation and errors associated with methods used in quantitating protein concentration. Here, a study was conducted to include the detection of five total and two housekeeping proteins to establish the integrity of our results. The panel included a 7-plex panel made of total Akt, mTOR, p70 S6 kinase, GSK-3β, PTEN, and two housekeeping proteins, β-actin and GAPDH. We found similar levels of these proteins in untreated and insulin-treated Hep G2 cells, providing stronger confidence in the observed changes in phosphorylation (Figure 3).
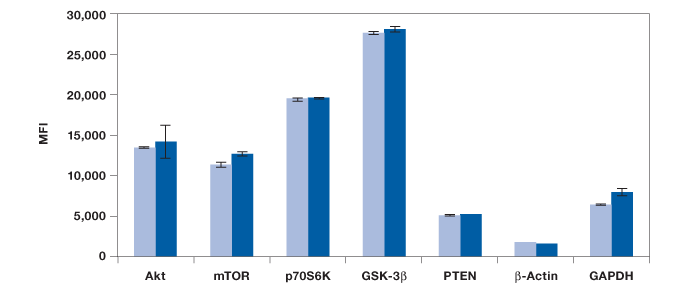
Fig. 3. Measurement of total proteins and housekeeping proteins. Seven assays were combined into one 7-plex panel to study total protein expression in untreated ( ) and insulin-treated cells (
) and insulin-treated cells ( ). MFI, median fluorescence intensity.
). MFI, median fluorescence intensity.
MAPK Signal Transduction Pathways in Growth and Stress
MAPK pathways are activated by a variety of external stimuli including growth factors, stress, and G protein–coupled receptors (GPCRs). These evolutionarily conserved kinases regulate fundamental cellular processes such as proliferation, survival, differentiation, and apoptosis in all eukaryotes (Cargnello and Roux 2011 and Morrison 2012).
A multiplex panel to study the MAPK signal transduction pathway was developed, which comprises nine key phosphoproteins of three MAPK cascades (Figure 4). To evaluate the performance of this panel, we studied two classic stimuli — a growth factor (in this case EGF) and stress (UV radiation) in SK-BR-3 and HEK-293 cell lines.
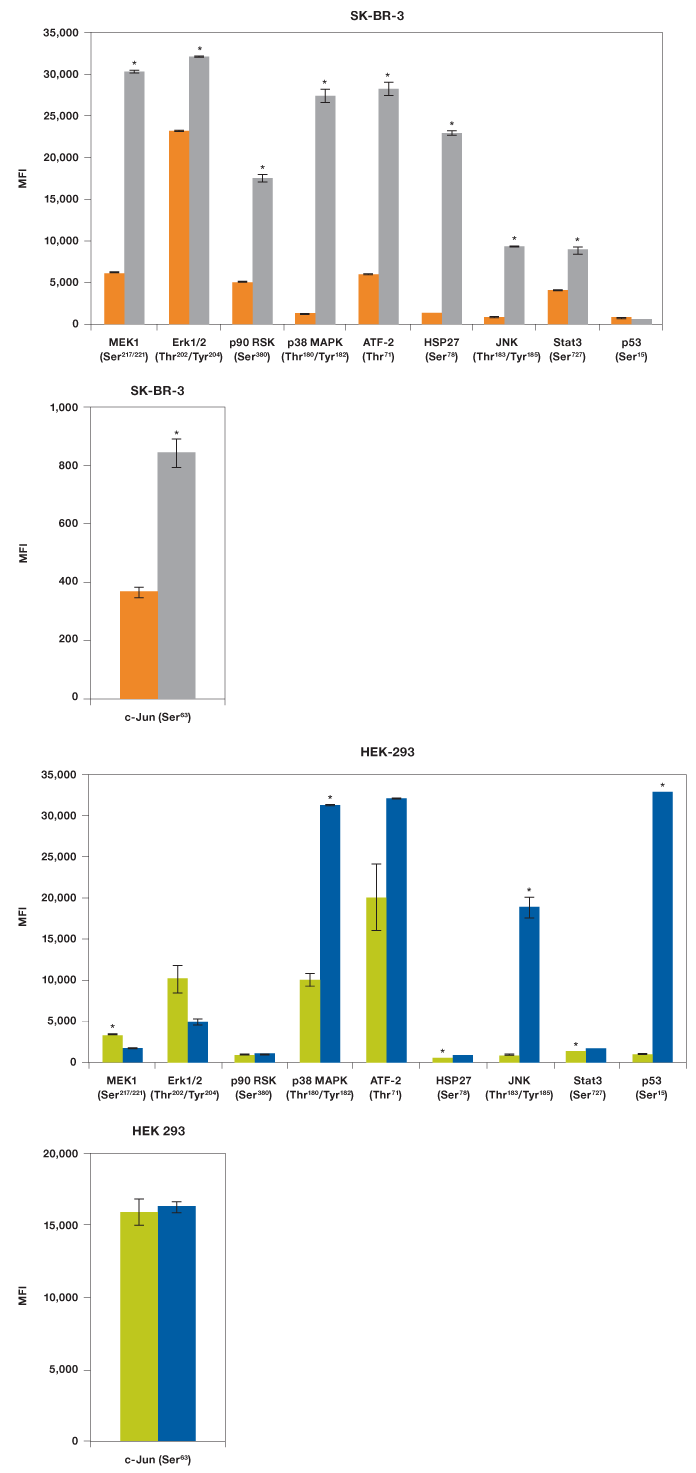
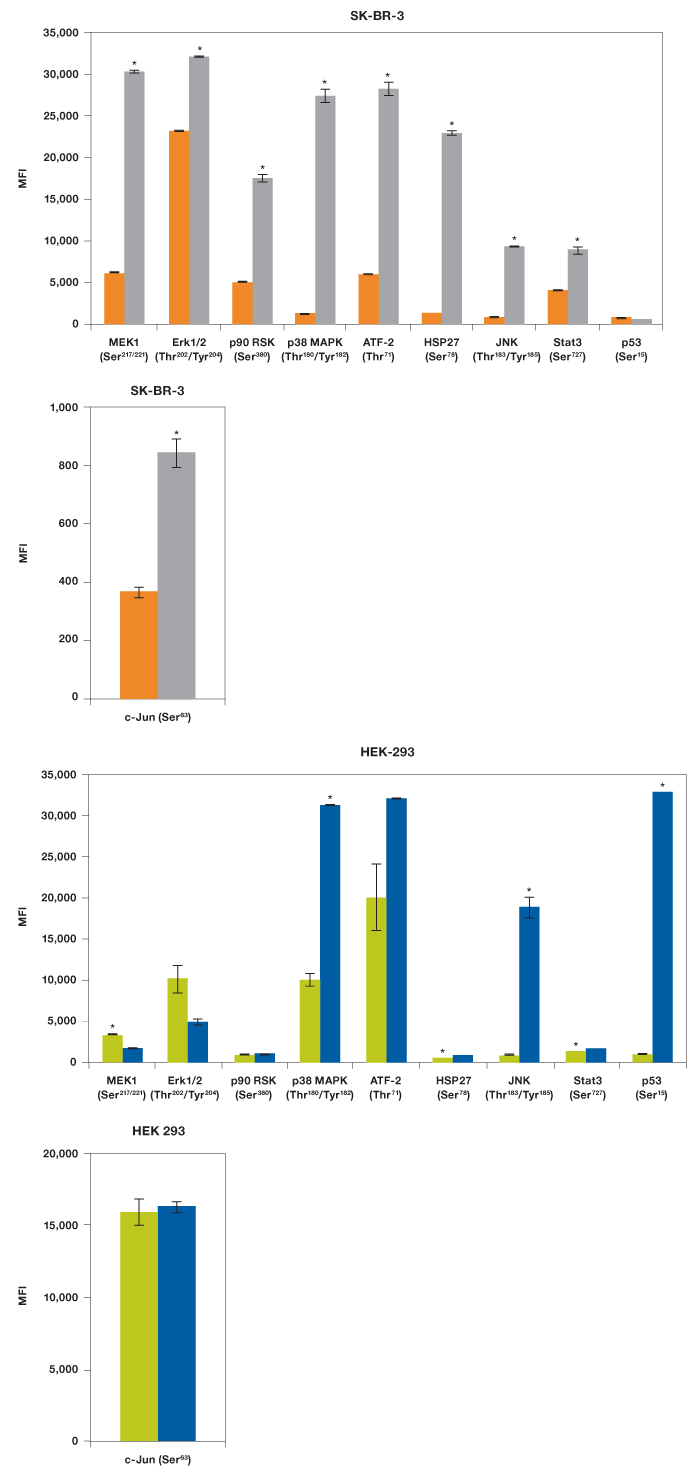
Our results showed that EGF was able to activate MEK1, all three MAPKs (Erk1/2, p38 MAPK, and JNK), two transcription factors (ATF-2 and Stat3), as well as p90 RSK and HSP27, but not p53 (Figure 5). As expected, UV irradiation resulted in a very different activation profile with strong phosphorylation of p38 MAPK and JNK, but not the MEK1-Erk1/2 module (Morrison 2012). Of the transcription factors, only p53 (Ser15) was strongly phosphorylated, which is consistent with its activation in response to signals arising from DNA-damaging UV radiation (She et al. 2000).
We also tested these lysates for phosphorylation of c-Jun with a singleplex assay (Figure 5). The c-Jun assay was not included in the panel as it cross-reacts with ATF-2, both proteins being components of the transcription factor activator protein-1 (AP-1). Treatment with EGF led to twofold activation of c-Jun (Ser63) while HEK-293 cells were characterized by high c-Jun (Ser63) expression even without radiation.
EGF receptor activation was confirmed with Bio-Plex Pro cell signaling EGF receptor (EGFR) (Tyr1068) and EGFR (Tyr1173) assays (Figure 6). Consistent with HER-2 activation by phosphorylated EGFR, we observed an increase in phosphorylation of HER-2 at tyrosine 1248 (Qian et al. 1994). All three assays resulted in high detection of receptor phosphorylation with a fivefold increase in treated cells. We further demonstrate that the levels of total HER-2 protein in treated cells did not change upon treatment with EGF, confirming posttranslational modification (Figure 6).
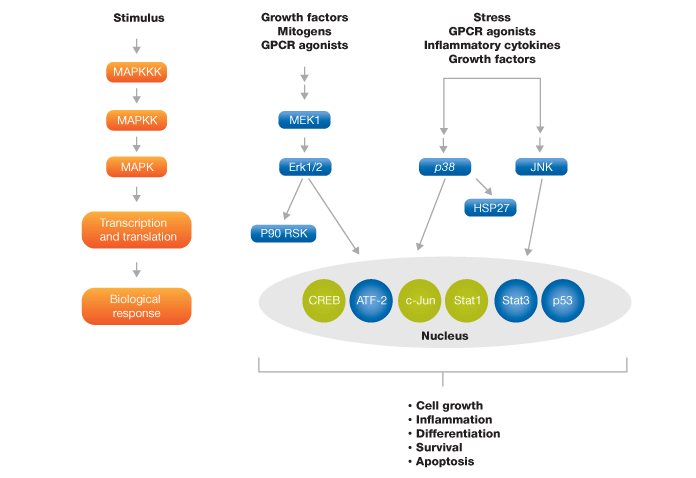
Fig. 4. Schematic representation of MAPK signaling pathway targets. The blue colored targets are part of the MAPK 9-plex panel and the green colored targets are available separately.
Fig. 5. EGF and UV activation of MAPK pathways. Using the Bio-Plex Pro cell signaling MAPK 9-plex panel and c-Jun (Ser63) singleplex assay, activation by phosphorylation of specific proteins was shown from SK-BR-3 cells untreated ( ) and EGF-treated (
) and EGF-treated ( ), and HEK-293 cells untreated (
), and HEK-293 cells untreated ( ) and UV-treated (
) and UV-treated ( ). *P
). *P
Fig. 6. EGFR and HER-2 activation in response to EGF. Untreated and EGF-treated SK-BR-3 0.2 mg/ml lysates (50 μl/well) were analyzed by Bio-Plex Pro cell signaling singleplex assays in duplicates. MFI, median fluorescence intensity.
Multiplexibility Studies
Changes in immunoassay mutliplexibility may result in variability in readout, which is mainly due to changes in antibody detection concentration and antibody cross-reactivity. The Bio-Plex Pro cell signaling assays were developed and optimized to minimize such effects. Assays with ≥10% cross-reactivity or interference are not recommended for multiplexing (see Methods). Here we describe two assay examples designed to detect interferences related to changes in multiplexibility. In the first study we tested to see if the addition of a target protein assay would affect the total assay outcome. IR-β (Tyr1146) assay was added to the Akt 8-plex panel to form a 9-plex panel (Figure 7). As expected from our cross-reactivity studies, we observed no effect from adding IR-β (Tyr1146) to the Akt 8-plex panel (Figure 7).
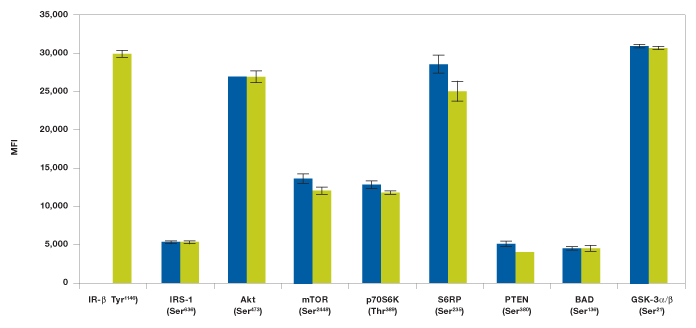
Fig. 7. Addition of IR-β assay to Akt 8-plex panel does not show interference. Insulin-stimulated Hep G2 cells were studied with a 9-plex panel ( ) made with Akt 8-plex panel and IR-β (Tyr1146) assay. The Akt 8-plex panel (
) made with Akt 8-plex panel and IR-β (Tyr1146) assay. The Akt 8-plex panel ( ) results are from Figure 2 and are added for easy comparison. MFI, median fluorescence intensity.
) results are from Figure 2 and are added for easy comparison. MFI, median fluorescence intensity.
In the second study, levels of phosphoproteins in EGF-treated SK-BR-3 cells were measured with either a singleplex assay or by the MAPK 9-plex panel to see if there was a difference between the outcomes. Consistent with our cross-reactivity data, no significant differences were observed when testing the same lysate in a singleplex format as compared to the 9-plex configuration (Figure 8).
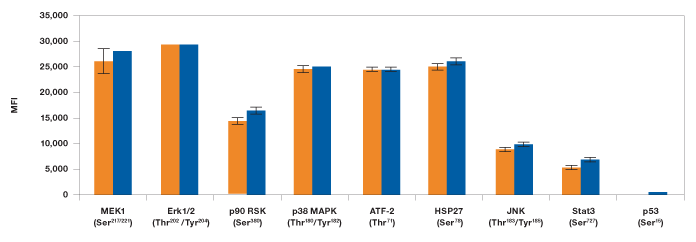
Fig. 8. Singleplex vs. multiplex comparison of MAPK panel assays. EGF-treated SK-BR-3 cell lysate was studied using the MAPK 9-plex panel ( ) and singleplex assays (
) and singleplex assays ( ). Protein phosphorylation was measured on the same plate in duplicates as described in Methods. MFI, median fluorescence intensity.
). Protein phosphorylation was measured on the same plate in duplicates as described in Methods. MFI, median fluorescence intensity.
Discussion
Akt is stimulated via receptor tyrosine kinases that are activated by ligands such as PDGF, EGF, FGF basic, VEGF, insulin, and IGF-1 as well as by G protein–coupled receptors and other stimuli like heat shock and H2O2. Attempts to characterize insulin and IGF-1 signaling led to the discovery that activation of Akt is accomplished by phosphorylation of residues Thr308 and Ser473 (Brazil and Hemmings 2001). The Bio-Plex Pro cell signaling Akt panel is designed for the sensitive detection of phosphorylated proteins involved in this signaling cascade. It should be noted that more assays relevant to the Akt pathway were developed but not included in this panel, like PI3K, CREB, Akt (Thr308), and p70 S6 kinase (Thr421/Ser424).
The utility of this panel was tested using Hep G2 cells treated with insulin, a well-established stimulator of the Akt signaling pathway. In clear agreement with published data, we observed the phosphorylation of IR-β, IRS-1, Akt (Thr308 and Ser473), mTOR, p70 S6 kinase (Thr389 and Thr421/Ser424), S6 ribosomal protein, BAD, and GSK-3α/β (Hemmings and Restucia 2012).
MAP kinases control three main cascades of signal transduction. The MAPK panel was configured for detection of nine kinases participating in these pathways. These nine kinases are involved in growth, survival, differentiation, development, inflammation, and apoptosis. In this study, we demonstrated the utility of this panel using two classic stimuli, EGF (growth factor) and UV (stress). Stimulation by EGF led to the phosphorylation of EGFR and the orphan receptor HER-2, supporting the EGFR/HER-2 heterodimerization model of HER-2 activation. Receptor activations were accompanied by phosphorylation of eight downstream effectors, MEK1, Erk1/2, p90 RSK, ATF-2, Stat3, p38 MAPK, JNK, and HSP27. As expected, UV treatment resulted in a markedly different activation profile, where MEK1, Erk1/2, and P90 RSK showed little or no response, and p38 MAPK and JNK were strongly phosphorylated. Consistent with previous reports, we observed a 30-fold increase in phosphorylated p53 (Ser15) in UV-treated cells.
Once a protein kinase is activated, its activity is subsequently downregulated through a variety of mechanisms, including phosphatase activity and protein degradation (Lu and Hunter 2009). These mechanisms can be deciphered using Bio-Plex Pro cell signaling assays. For example, decline in phosphorylation with unchanged total protein level would suggest downregulation by a phosphatase while a decline in protein content would suggest protein degradation. In addition, total protein detection is relevant for confirming sample consistency, preparation, and loading. These sample quality controls can also be addressed with Bio-Plex Pro assays developed for the detection of housekeeping proteins β-actin and GAPDH.
It should be noted that all the Bio-Plex assays described in this publication were performed using less than two 96-well plates, including positive and control lysates, and can be accomplished in 24–48 hours by one investigator. Thus, by measuring multiple markers simultaneously, studies can be done at reduced time, cost, and sample volume compared to more traditional methods such as ELISA and western blotting. While the studies presented here focused on the effects of external activators, the same assays can be used to quickly generate a wealth of information on different aspects of signal transduction such as the basal phosphorylation levels in cells, effective concentration of mitogens, and effects of kinase inhibitors on signal transduction pathways.
The studies presented here are in agreement with published data and confirm the exceptional sensitivity and specificity of the Bio-Plex Pro cell signaling assays in mapping intricate Akt and MAPK pathways. Furthermore, these results complement previous studies of small molecule kinase inhibitors and signal transduction pathways in human cancer tissue (Peretz et al. 2013a and 2013b).
References
Boulanger LM et al. (2001). Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol 11, 568–578.
Brazil PD and Hemmings AB (2001). Ten years of protein kinase B signaling: A hard Akt to follow. Trends Biochem Sci 26, 657–664.
Cargnello M and Roux PP (2011). Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol and Mol Biol Rev 75, 50–83.
Chang L and Karin M (2001). Mammalian MAP kinase signaling cascades. Nature 410, 37–40.
Dale E et al. (2008). Second-generation multiplex immunoassays. BioRadiations 125, 16–21.
Dummler AB and Hemmings AB (2004). Protein kinase B. Encyclopedia of Biological Chemistry 3, 516–522.
Hemmings AB and Restuccia FD (2012). PI3K-PKB/AKT pathway. Cold Spring Harb Perspect Biol 4, 1–3.
Lu Z and Hunter T (2009). Degradation of activated protein kinases by ubiquitination. Annu Rev Biochem 78, 435–475.
Manning DB et al. (2007). AKT/PKB Signaling: Navigating Downstream. Cell 129, 1261–1274.
Manning G et al. (2002). The protein kinase complement of the human genome. Science 298, 1912–1934.
Morrison KD (2012). MAP kinase pathways. Cold Spring Harb Perspect Biol 4, 1–5.
Peretz D et al. (2013a). Validation and application of Bio-Plex Pro cell signaling assays. Bio-Rad Bulletin 6331.
Peretz D et al. (2013b). Profiling of cell signaling pathways and angiogenesis biomarkers in human cancer tissue. Bio-Rad Bulletin 6330.
Qian X et al. (1994). Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: A mechanism of interreceptor kinase activation and transphosphorylation. PNAS 91, 1500–1504.
She Q-B et al. (2000). ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chemistry 275, 20444–20449.
Yates D (2011). Development: Directing development through phosphorylation. Nat Rev Neurosci 12, 248–249.
Yeung D et al. (2011). Multiplex analysis of rat cytokines and diabetes biomarkers using Bio-Plex Pro rat cytokine and diabetes assays. Bio-Rad Bulletin 6119.
Zhou H and Geng W (2011). Analysis of murine Th17 cytokine profiles using the Bio-Plex Pro mouse Th17 panel. Bio-Rad Bulletin 6118.
The Bio-Plex suspension array system includes fluorescently labeled microspheres and instrumentation licensed to Bio-Rad Laboratories, Inc. by the Luminex Corporation.
xMAP and Luminex are trademarks of the Luminex Corporation.

CST antibodies developed and validated for Bio-Plex phosphoprotein, and total target assays.