Abstract
This report demonstrates that the TC10 automated cell counter is a suitable alternative to the Coulter Counter and hemocytometer for the preparation of custom Bio-Plex assays.
Introduction
The Bio-Plex suspension array system is a powerful tool for measuring analyte concentration. At the core of Bio-Rad’s Bio-Plex Pro™ assays are magnetic carboxylated beads, microscopic spheres capable of carrying detection molecules, typically antibodies. While a variety of validated assays are currently available, many Bio-Plex system users are developing custom assays. When developing a custom assay it is essential to determine bead concentration accurately and consistently to ensure that uniform quantities of antibody are used in the bead-antibody conjugation step. Suboptimal conjugation will affect the sensitivity and dynamic range of the assay.
A method commonly used to determine bead concentration is particle counting using a a Coulter-based flow cytometry device such as a Coulter Counter. Flow cytometry-based devices measure particles in a stream using impedance technology. A precisely controlled flow allows volumetric measurements and estimation of bead concentration within the sample. While the technology is capable, there are some disadvantages: Coulter Counters are relatively expensive, the instruments must be calibrated regularly, they are prone to clogging, and there is no image available to confirm count accuracy.
Another frequently used method for bead counting is manual counting using a hemocytometer. Manual counting suffers from a variety issues: it is tedious and laborious and results are prone to error due to user subjectivity (Hefner et al. 2010). The advantage is that hemocytometers are readily available, inexpensive, and are used with standard laboratory equipment.
We demonstrate that while the TC10 cell counter was optimized for cell counting (Hefner et al. 2010, Hsuing et al. 2010), the instrument is also well suited to counting microspheres used in the Bio-Plex suspension array system. In addition, the TC10 offers a variety of advantages over the Coulter Counter and the hemocytometer.
Materials and Methods
Preparation of Beads
Raw polystyrene and magnetic beads were prepared in a range of concentrations from stock solutions of 1.25 x 107 beads/ml. Six dilutions were prepared for each bead type by vortexing the stock tube of beads for 30 seconds to fully resuspend the beads and then by diluting the bead stock into PBS.
Hemocytometer Counting
Bead counts were performed using C-Chip disposable hemocytometers (In CYTO) and an Olympus IX70 inverted microscope fitted with a Retiga EXi CCD camera from QImaging. Counting was repeated three times at each concentration and the number of beads was calculated by taking the mean number of beads from the 4 corner cells of the Neubauer grid.
Beckman Coulter Counting
The beads were counted using a Beckman Coulter Z2 Coulter Counter. The counter was gated for 4–8 µm for the polystyrene beads and 5–9 µm for the magnetic beads. The beads were diluted in IsoFlow sheath fluid (Beckman Coulter). Each count was repeated three times using the automated count average function.
TC10 Automated Cell Counting
Bead counts were performed using a TC10 automated cell counter (Bio-Rad) by loading 10 µl of each bead solution into a dual chamber TC10 sample slide (Bio-Rad). Each bead solution was counted three times and averaged.
The same person conducted all of the counting using the different techniques and worked from the same tubes of beads for consistency. Each bead preparation was counted three times to allow an average and standard deviation to be determined.
Results
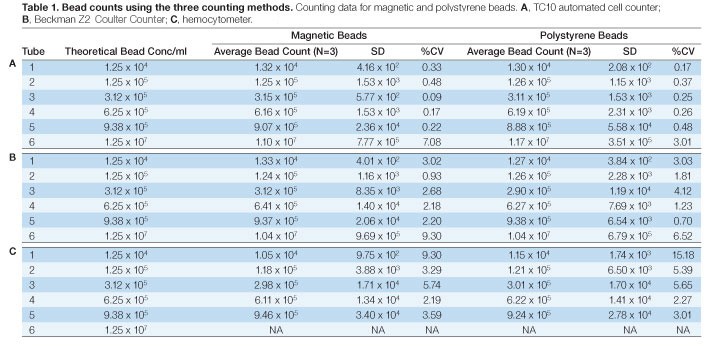
Six concentrations from 1.25 x 104 to 9.38 x 105 beads/ml of uncoupled Bio-Plex beads, both polystyrene (5.6 µm ± 0.2 µm) and magnetic (6.5 µm ± 0.2 µm), were measured using the TC10 automated cell counter, a hemocytometer and a Coulter Counter. All three methods produced comparable results that matched the expected concentration (Fig 1). Except for the 9.38 x 105 beads/ml concentration of polystyrene beads, the standard deviations for data collected on the TC10 automated cell counter were equal to or lower than for the data collected using the Coulter Counter and the hemocytometer (Tables 1A–1C). The bead concentration in figure 1 represents dilutions from a stock concentration of 1.25 x 107 beads/ml.
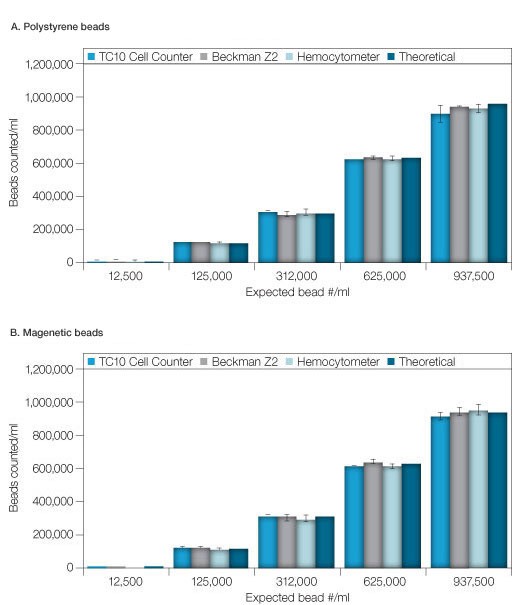
Figure 1. Comparison of counts of various bead concentrations using the various methods. A, polystyrene beads. B, magnetic beads. The theoretical concentration was determined from dilutions of a stock/validated solution. Error bars = 1 standard deviation.
The bead stock solution (1.25 x 107 beads/ml), was also counted using the TC10 automated cell counter and the Coulter Counter. Figure 2 is a representative image captured with the TC10 automated cell counter of a high-concentration solution of beads with bead identifier annotation. As for the diluted bead solutions, results were comparable between the two methods with precision favoring the TC10 automated cell counter (Figures 3A and 3B). It should be noted that hemocytometer counts using the undiluted beads were not attempted due to the difficulty associated with counting extremely high concentrations with this method.
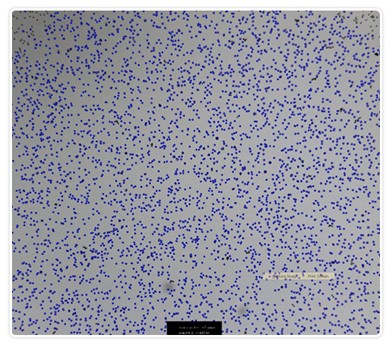
Figure 2. Representative image of a high-concentration solution of beads. Image was taken using the TC10 automated cell counter. The blue dots indicate beads that were counted.
Conclusion
The TC10 automated cell counter accurately and precisely counted both polystyrene and magnetic Bio-Plex beads over a wide range of concentrations. A significant advantage the TC10 automated cell counter has over the alternative approaches is the speed at which the counts can be achieved and its ease of use. Both the Coulter Counter and the hemocytometer require significant input from the operator to prepare the samples and then operate the machine. The time to count the beads on the hemocytometer is concentration-dependent with the lowest and highest concentrations taking up to 4–5 minutes per sample to obtain an accurate count. Also the autofocus function of the TC10 automated counter makes it quicker and easier to use compared with the hemacytometer. The TC10 automated cell counter is an excellent option for counting beads used in the Bio-Plex suspension array system.
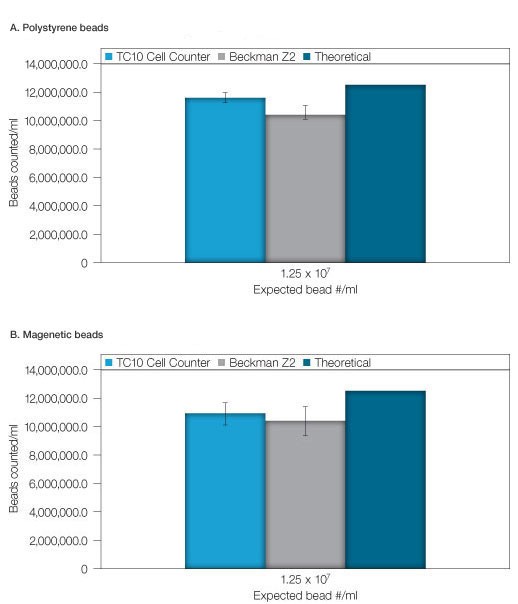
Figure 3. High concentration bead counting. Count of a high concentration bead solution was performed on the TC10 cell counter, the Beckman Z2 Coulter Counter and a hemocytometer. A, polystyrene beads. B, magnetic beads. The theoretical concentration was determined from a validated stock solution. Error bars = 1 standard deviation.
References
Hefner et. al. (2010). Comparison of count reproducibility, accuracy, and time to results between a hemocytometer and the TC10 automated cell counter. Bio-Rad Bulletin 6003.
Hsiung et. al. (2010). Multifocal plane analysis is essential for accurate cell viability assessment using an automated cell counter. Bio-Rad Bulletin 6011.
Coulter Counter is a trademark of Beckman Coulter, Inc.
The Bio-Plex suspension array system includes fluorescently labeled microspheres and instrumentation licensed to Bio-Rad Laboratories, Inc. by the Luminex Corporation.
C-Chip is a trademark of the Digital Bio Technology, Co., Ltd.
Isoflow is a trademark of Beckman Coulter.

